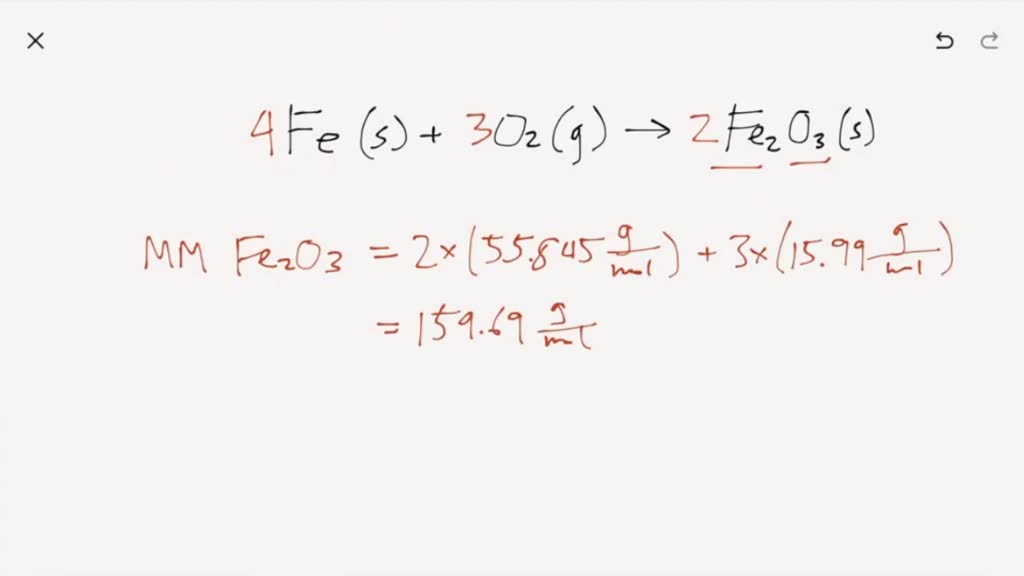Iron Ii Oxide + Oxygen Balanced Equation . Upon reacting with oxygen, iron will be. 2f e(s) +3o2(g) → 2f e2o3(s) this reaction can be. H 2 + o 2 = 2h 2 o now, there are 4 h atoms on the right side, so we. Here are the equations for the reaction. 1 feo = 1 fe + 1 o 2 for each element, we check if the. F e(s) + o2(g) → f e2o3(s)? Iron can react with oxygen to form two of its oxides, iron (ii, iii) oxide and iron (iii) oxide. How do you balance and translate this reaction: Balance the oxygen atoms by placing a coefficient of 2 in front of h 2 o: First, we set all coefficients to 1: Iron (iii) oxide reacts with chlorine gas to give iron (iii) chloride and oxygen gas, according to the equation shown below; 1 feo = 1 fe + 1 o 2 pour chaque élément, on vérifie si le nombre d’atomes est équilibré des deux côtés de l’équation. Iron + dioxygen = iron(ii) oxide. Let's balance this equation using the inspection method. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o.
from www.numerade.com
Iron + dioxygen = iron(ii) oxide. Balance the oxygen atoms by placing a coefficient of 2 in front of h 2 o: Iron can react with oxygen to form two of its oxides, iron (ii, iii) oxide and iron (iii) oxide. How do you balance and translate this reaction: H 2 + o 2 = 2h 2 o now, there are 4 h atoms on the right side, so we. F e(s) + o2(g) → f e2o3(s)? 2f e(s) +3o2(g) → 2f e2o3(s) this reaction can be. First, we set all coefficients to 1: 1 feo = 1 fe + 1 o 2 for each element, we check if the. Iron (iii) oxide reacts with chlorine gas to give iron (iii) chloride and oxygen gas, according to the equation shown below;
SOLVEDIron metal reacts with oxygen to give iron(III) oxide, Fe2 O3 (a
Iron Ii Oxide + Oxygen Balanced Equation F e(s) + o2(g) → f e2o3(s)? Iron can react with oxygen to form two of its oxides, iron (ii, iii) oxide and iron (iii) oxide. Iron (iii) oxide reacts with chlorine gas to give iron (iii) chloride and oxygen gas, according to the equation shown below; Here are the equations for the reaction. Upon reacting with oxygen, iron will be. Iron + dioxygen = iron(ii) oxide. 1 feo = 1 fe + 1 o 2 pour chaque élément, on vérifie si le nombre d’atomes est équilibré des deux côtés de l’équation. Let's balance this equation using the inspection method. F e(s) + o2(g) → f e2o3(s)? First, we set all coefficients to 1: Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o. H 2 + o 2 = 2h 2 o now, there are 4 h atoms on the right side, so we. Balance the oxygen atoms by placing a coefficient of 2 in front of h 2 o: How do you balance and translate this reaction: 2f e(s) +3o2(g) → 2f e2o3(s) this reaction can be. 1 feo = 1 fe + 1 o 2 for each element, we check if the.
From www.numerade.com
SOLVED Solid iron(II) hydroxide to form solid iron(II Iron Ii Oxide + Oxygen Balanced Equation Iron + dioxygen = iron(ii) oxide. Upon reacting with oxygen, iron will be. H 2 + o 2 = 2h 2 o now, there are 4 h atoms on the right side, so we. Balance the oxygen atoms by placing a coefficient of 2 in front of h 2 o: F e(s) + o2(g) → f e2o3(s)? 1 feo =. Iron Ii Oxide + Oxygen Balanced Equation.
From www.numerade.com
SOLVED(i) Calculate oxidation number of (a) Fe in FezO3 (b) Pin KzHPO4 Iron Ii Oxide + Oxygen Balanced Equation 2f e(s) +3o2(g) → 2f e2o3(s) this reaction can be. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o. Let's balance this equation using the inspection method. 1 feo = 1 fe + 1 o 2 for each element, we check if the. Upon reacting with oxygen, iron. Iron Ii Oxide + Oxygen Balanced Equation.
From www.youtube.com
Redox Reaction Oxidation of Iron (II) to Iron (III) Redox Iron Ii Oxide + Oxygen Balanced Equation 1 feo = 1 fe + 1 o 2 for each element, we check if the. F e(s) + o2(g) → f e2o3(s)? 2f e(s) +3o2(g) → 2f e2o3(s) this reaction can be. 1 feo = 1 fe + 1 o 2 pour chaque élément, on vérifie si le nombre d’atomes est équilibré des deux côtés de l’équation. Balance the. Iron Ii Oxide + Oxygen Balanced Equation.
From www.numerade.com
SOLVEDWrite the balanced equation for the preparation of iron(III Iron Ii Oxide + Oxygen Balanced Equation 1 feo = 1 fe + 1 o 2 for each element, we check if the. How do you balance and translate this reaction: 1 feo = 1 fe + 1 o 2 pour chaque élément, on vérifie si le nombre d’atomes est équilibré des deux côtés de l’équation. Iron can react with oxygen to form two of its oxides,. Iron Ii Oxide + Oxygen Balanced Equation.
From www.numerade.com
SOLVED When the following molecular equation is balanced using the Iron Ii Oxide + Oxygen Balanced Equation First, we set all coefficients to 1: H 2 + o 2 = 2h 2 o now, there are 4 h atoms on the right side, so we. Balance the oxygen atoms by placing a coefficient of 2 in front of h 2 o: Here are the equations for the reaction. Iron (iii) oxide reacts with chlorine gas to give. Iron Ii Oxide + Oxygen Balanced Equation.
From www.numerade.com
SOLVEDIron metal reacts with oxygen to give iron(III) oxide, Fe2 O3 (a Iron Ii Oxide + Oxygen Balanced Equation H 2 + o 2 = 2h 2 o now, there are 4 h atoms on the right side, so we. Iron + dioxygen = iron(ii) oxide. Iron (iii) oxide reacts with chlorine gas to give iron (iii) chloride and oxygen gas, according to the equation shown below; Fe + o2 = feo is a synthesis reaction where two moles. Iron Ii Oxide + Oxygen Balanced Equation.
From www.chegg.com
Solved 5. Iron II oxide reacts violently and exothermically Iron Ii Oxide + Oxygen Balanced Equation First, we set all coefficients to 1: Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o. Iron can react with oxygen to form two of its oxides, iron (ii, iii) oxide and iron (iii) oxide. Upon reacting with oxygen, iron will be. Iron (iii) oxide reacts with chlorine. Iron Ii Oxide + Oxygen Balanced Equation.
From www.chegg.com
Solved Iron (II) oxide reacts with carbon monoxide to form Iron Ii Oxide + Oxygen Balanced Equation 2f e(s) +3o2(g) → 2f e2o3(s) this reaction can be. F e(s) + o2(g) → f e2o3(s)? Here are the equations for the reaction. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o. Iron can react with oxygen to form two of its oxides, iron (ii, iii) oxide. Iron Ii Oxide + Oxygen Balanced Equation.
From www.teachoo.com
Reaction of Metals and Nonmetals with Oxygen Concepts Iron Ii Oxide + Oxygen Balanced Equation Here are the equations for the reaction. Iron can react with oxygen to form two of its oxides, iron (ii, iii) oxide and iron (iii) oxide. 2f e(s) +3o2(g) → 2f e2o3(s) this reaction can be. Upon reacting with oxygen, iron will be. First, we set all coefficients to 1: 1 feo = 1 fe + 1 o 2 pour. Iron Ii Oxide + Oxygen Balanced Equation.
From www.thoughtco.com
Easy Steps for Balancing Chemical Equations Iron Ii Oxide + Oxygen Balanced Equation Here are the equations for the reaction. Upon reacting with oxygen, iron will be. 1 feo = 1 fe + 1 o 2 for each element, we check if the. F e(s) + o2(g) → f e2o3(s)? 2f e(s) +3o2(g) → 2f e2o3(s) this reaction can be. H 2 + o 2 = 2h 2 o now, there are 4. Iron Ii Oxide + Oxygen Balanced Equation.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation ID3552860 Iron Ii Oxide + Oxygen Balanced Equation 2f e(s) +3o2(g) → 2f e2o3(s) this reaction can be. Upon reacting with oxygen, iron will be. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o. F e(s) + o2(g) → f e2o3(s)? How do you balance and translate this reaction: Iron + dioxygen = iron(ii) oxide. Iron. Iron Ii Oxide + Oxygen Balanced Equation.
From www.youtube.com
How to write the formula for iron (II) oxide YouTube Iron Ii Oxide + Oxygen Balanced Equation 1 feo = 1 fe + 1 o 2 pour chaque élément, on vérifie si le nombre d’atomes est équilibré des deux côtés de l’équation. Iron can react with oxygen to form two of its oxides, iron (ii, iii) oxide and iron (iii) oxide. 1 feo = 1 fe + 1 o 2 for each element, we check if the.. Iron Ii Oxide + Oxygen Balanced Equation.
From wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry Iron Ii Oxide + Oxygen Balanced Equation Iron + dioxygen = iron(ii) oxide. Balance the oxygen atoms by placing a coefficient of 2 in front of h 2 o: First, we set all coefficients to 1: Iron (iii) oxide reacts with chlorine gas to give iron (iii) chloride and oxygen gas, according to the equation shown below; Fe + o2 = feo is a synthesis reaction where. Iron Ii Oxide + Oxygen Balanced Equation.
From www.thoughtco.com
Easy Steps for Balancing Chemical Equations Iron Ii Oxide + Oxygen Balanced Equation H 2 + o 2 = 2h 2 o now, there are 4 h atoms on the right side, so we. F e(s) + o2(g) → f e2o3(s)? How do you balance and translate this reaction: 1 feo = 1 fe + 1 o 2 pour chaque élément, on vérifie si le nombre d’atomes est équilibré des deux côtés de. Iron Ii Oxide + Oxygen Balanced Equation.
From www.youtube.com
Balancing Equations Iron and Oxygen YouTube Iron Ii Oxide + Oxygen Balanced Equation Iron + dioxygen = iron(ii) oxide. Upon reacting with oxygen, iron will be. H 2 + o 2 = 2h 2 o now, there are 4 h atoms on the right side, so we. Balance the oxygen atoms by placing a coefficient of 2 in front of h 2 o: How do you balance and translate this reaction: Here are. Iron Ii Oxide + Oxygen Balanced Equation.
From www.numerade.com
SOLVED of iron with oxygen gas and water produces iron (III) oxide (a Iron Ii Oxide + Oxygen Balanced Equation Upon reacting with oxygen, iron will be. 2f e(s) +3o2(g) → 2f e2o3(s) this reaction can be. F e(s) + o2(g) → f e2o3(s)? Balance the oxygen atoms by placing a coefficient of 2 in front of h 2 o: Let's balance this equation using the inspection method. Iron (iii) oxide reacts with chlorine gas to give iron (iii) chloride. Iron Ii Oxide + Oxygen Balanced Equation.
From www.vrogue.co
What Is Formed In The Reaction Between Iron And Oxyge vrogue.co Iron Ii Oxide + Oxygen Balanced Equation Iron (iii) oxide reacts with chlorine gas to give iron (iii) chloride and oxygen gas, according to the equation shown below; Iron + dioxygen = iron(ii) oxide. H 2 + o 2 = 2h 2 o now, there are 4 h atoms on the right side, so we. How do you balance and translate this reaction: Here are the equations. Iron Ii Oxide + Oxygen Balanced Equation.
From www.slideserve.com
PPT Reacting metals with oxygen PowerPoint Presentation, free Iron Ii Oxide + Oxygen Balanced Equation F e(s) + o2(g) → f e2o3(s)? Balance the oxygen atoms by placing a coefficient of 2 in front of h 2 o: Here are the equations for the reaction. H 2 + o 2 = 2h 2 o now, there are 4 h atoms on the right side, so we. Iron + dioxygen = iron(ii) oxide. Iron can react. Iron Ii Oxide + Oxygen Balanced Equation.
From testbook.com
Iron (II) Oxide Formula Structure, Preparation & Properties Iron Ii Oxide + Oxygen Balanced Equation Iron (iii) oxide reacts with chlorine gas to give iron (iii) chloride and oxygen gas, according to the equation shown below; 1 feo = 1 fe + 1 o 2 for each element, we check if the. 1 feo = 1 fe + 1 o 2 pour chaque élément, on vérifie si le nombre d’atomes est équilibré des deux côtés. Iron Ii Oxide + Oxygen Balanced Equation.
From www.slideserve.com
PPT Unit 7 Chemical Equations PowerPoint Presentation, free download Iron Ii Oxide + Oxygen Balanced Equation H 2 + o 2 = 2h 2 o now, there are 4 h atoms on the right side, so we. Here are the equations for the reaction. Iron + dioxygen = iron(ii) oxide. First, we set all coefficients to 1: 1 feo = 1 fe + 1 o 2 for each element, we check if the. Iron can react. Iron Ii Oxide + Oxygen Balanced Equation.
From www.chegg.com
Solved 2. Write a balanced chemical equation for the Iron Ii Oxide + Oxygen Balanced Equation 2f e(s) +3o2(g) → 2f e2o3(s) this reaction can be. How do you balance and translate this reaction: Upon reacting with oxygen, iron will be. Iron (iii) oxide reacts with chlorine gas to give iron (iii) chloride and oxygen gas, according to the equation shown below; Let's balance this equation using the inspection method. Balance the oxygen atoms by placing. Iron Ii Oxide + Oxygen Balanced Equation.
From www.chegg.com
Solved 3 Powdered aluminum and iron(II) oxide react with a Iron Ii Oxide + Oxygen Balanced Equation First, we set all coefficients to 1: 1 feo = 1 fe + 1 o 2 for each element, we check if the. 2f e(s) +3o2(g) → 2f e2o3(s) this reaction can be. Here are the equations for the reaction. Let's balance this equation using the inspection method. Iron can react with oxygen to form two of its oxides, iron. Iron Ii Oxide + Oxygen Balanced Equation.
From www.numerade.com
SOLVED Iron(III) oxide, Fe2O3, is a result of the reaction of iron Iron Ii Oxide + Oxygen Balanced Equation F e(s) + o2(g) → f e2o3(s)? 2f e(s) +3o2(g) → 2f e2o3(s) this reaction can be. How do you balance and translate this reaction: 1 feo = 1 fe + 1 o 2 for each element, we check if the. Upon reacting with oxygen, iron will be. Balance the oxygen atoms by placing a coefficient of 2 in front. Iron Ii Oxide + Oxygen Balanced Equation.
From www.numerade.com
SOLVED 'In this laboratory experiment; two oxides of iron can form Iron Ii Oxide + Oxygen Balanced Equation Iron can react with oxygen to form two of its oxides, iron (ii, iii) oxide and iron (iii) oxide. Iron (iii) oxide reacts with chlorine gas to give iron (iii) chloride and oxygen gas, according to the equation shown below; Here are the equations for the reaction. Upon reacting with oxygen, iron will be. F e(s) + o2(g) → f. Iron Ii Oxide + Oxygen Balanced Equation.
From gbu-taganskij.ru
SOLVED Iron Reacts With Oxygen To Form Iron Oxide Name The, 53 OFF Iron Ii Oxide + Oxygen Balanced Equation 1 feo = 1 fe + 1 o 2 pour chaque élément, on vérifie si le nombre d’atomes est équilibré des deux côtés de l’équation. 2f e(s) +3o2(g) → 2f e2o3(s) this reaction can be. Here are the equations for the reaction. First, we set all coefficients to 1: Let's balance this equation using the inspection method. How do you. Iron Ii Oxide + Oxygen Balanced Equation.
From www.numerade.com
SOLVEDThe mineral (Fe3 O4) is whereas iron(II Iron Ii Oxide + Oxygen Balanced Equation F e(s) + o2(g) → f e2o3(s)? How do you balance and translate this reaction: H 2 + o 2 = 2h 2 o now, there are 4 h atoms on the right side, so we. Iron (iii) oxide reacts with chlorine gas to give iron (iii) chloride and oxygen gas, according to the equation shown below; Iron + dioxygen. Iron Ii Oxide + Oxygen Balanced Equation.
From ar.inspiredpencil.com
Structural Formula Of Iron Oxide Iron Ii Oxide + Oxygen Balanced Equation Here are the equations for the reaction. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o. Balance the oxygen atoms by placing a coefficient of 2 in front of h 2 o: 2f e(s) +3o2(g) → 2f e2o3(s) this reaction can be. Iron + dioxygen = iron(ii) oxide.. Iron Ii Oxide + Oxygen Balanced Equation.
From www.youtube.com
How to Balance Fe + O2 + H2O = Fe2O3 . xH2O (Iron + Oxygen gas + Water Iron Ii Oxide + Oxygen Balanced Equation Iron (iii) oxide reacts with chlorine gas to give iron (iii) chloride and oxygen gas, according to the equation shown below; 2f e(s) +3o2(g) → 2f e2o3(s) this reaction can be. Iron + dioxygen = iron(ii) oxide. F e(s) + o2(g) → f e2o3(s)? 1 feo = 1 fe + 1 o 2 for each element, we check if the.. Iron Ii Oxide + Oxygen Balanced Equation.
From www.pw.live
Iron II Oxide Formula Structure, Properties, Uses Iron Ii Oxide + Oxygen Balanced Equation Iron (iii) oxide reacts with chlorine gas to give iron (iii) chloride and oxygen gas, according to the equation shown below; 1 feo = 1 fe + 1 o 2 for each element, we check if the. F e(s) + o2(g) → f e2o3(s)? Here are the equations for the reaction. Iron + dioxygen = iron(ii) oxide. First, we set. Iron Ii Oxide + Oxygen Balanced Equation.
From www.numerade.com
SOLVEDMarine Chemistry of Iron On the seafloor, solid iron(II) oxide Iron Ii Oxide + Oxygen Balanced Equation How do you balance and translate this reaction: Let's balance this equation using the inspection method. Iron (iii) oxide reacts with chlorine gas to give iron (iii) chloride and oxygen gas, according to the equation shown below; 2f e(s) +3o2(g) → 2f e2o3(s) this reaction can be. 1 feo = 1 fe + 1 o 2 for each element, we. Iron Ii Oxide + Oxygen Balanced Equation.
From www.youtube.com
How to write the equation for FeO + H2O Iron (II) oxide + Water YouTube Iron Ii Oxide + Oxygen Balanced Equation 1 feo = 1 fe + 1 o 2 for each element, we check if the. Iron can react with oxygen to form two of its oxides, iron (ii, iii) oxide and iron (iii) oxide. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole of dioxygen [o. Upon reacting with oxygen,. Iron Ii Oxide + Oxygen Balanced Equation.
From vhgessaynfc.web.fc2.com
Write a balanced equation for iron iii oxide Iron Ii Oxide + Oxygen Balanced Equation H 2 + o 2 = 2h 2 o now, there are 4 h atoms on the right side, so we. 1 feo = 1 fe + 1 o 2 for each element, we check if the. 2f e(s) +3o2(g) → 2f e2o3(s) this reaction can be. Iron + dioxygen = iron(ii) oxide. First, we set all coefficients to 1:. Iron Ii Oxide + Oxygen Balanced Equation.
From priscillanewslara.blogspot.com
Molar Mass of Iron Ii Oxide Iron Ii Oxide + Oxygen Balanced Equation How do you balance and translate this reaction: First, we set all coefficients to 1: H 2 + o 2 = 2h 2 o now, there are 4 h atoms on the right side, so we. Iron + dioxygen = iron(ii) oxide. Fe + o2 = feo is a synthesis reaction where two moles of iron [fe] and one mole. Iron Ii Oxide + Oxygen Balanced Equation.
From www.numerade.com
SOLVED 1. Iron reacts with oxygen to produce iron(III)oxide =. The Iron Ii Oxide + Oxygen Balanced Equation First, we set all coefficients to 1: F e(s) + o2(g) → f e2o3(s)? Iron (iii) oxide reacts with chlorine gas to give iron (iii) chloride and oxygen gas, according to the equation shown below; Iron can react with oxygen to form two of its oxides, iron (ii, iii) oxide and iron (iii) oxide. Fe + o2 = feo is. Iron Ii Oxide + Oxygen Balanced Equation.
From www.youtube.com
How to Write the Formula for Iron (II) oxide YouTube Iron Ii Oxide + Oxygen Balanced Equation 1 feo = 1 fe + 1 o 2 pour chaque élément, on vérifie si le nombre d’atomes est équilibré des deux côtés de l’équation. First, we set all coefficients to 1: F e(s) + o2(g) → f e2o3(s)? Balance the oxygen atoms by placing a coefficient of 2 in front of h 2 o: Let's balance this equation using. Iron Ii Oxide + Oxygen Balanced Equation.