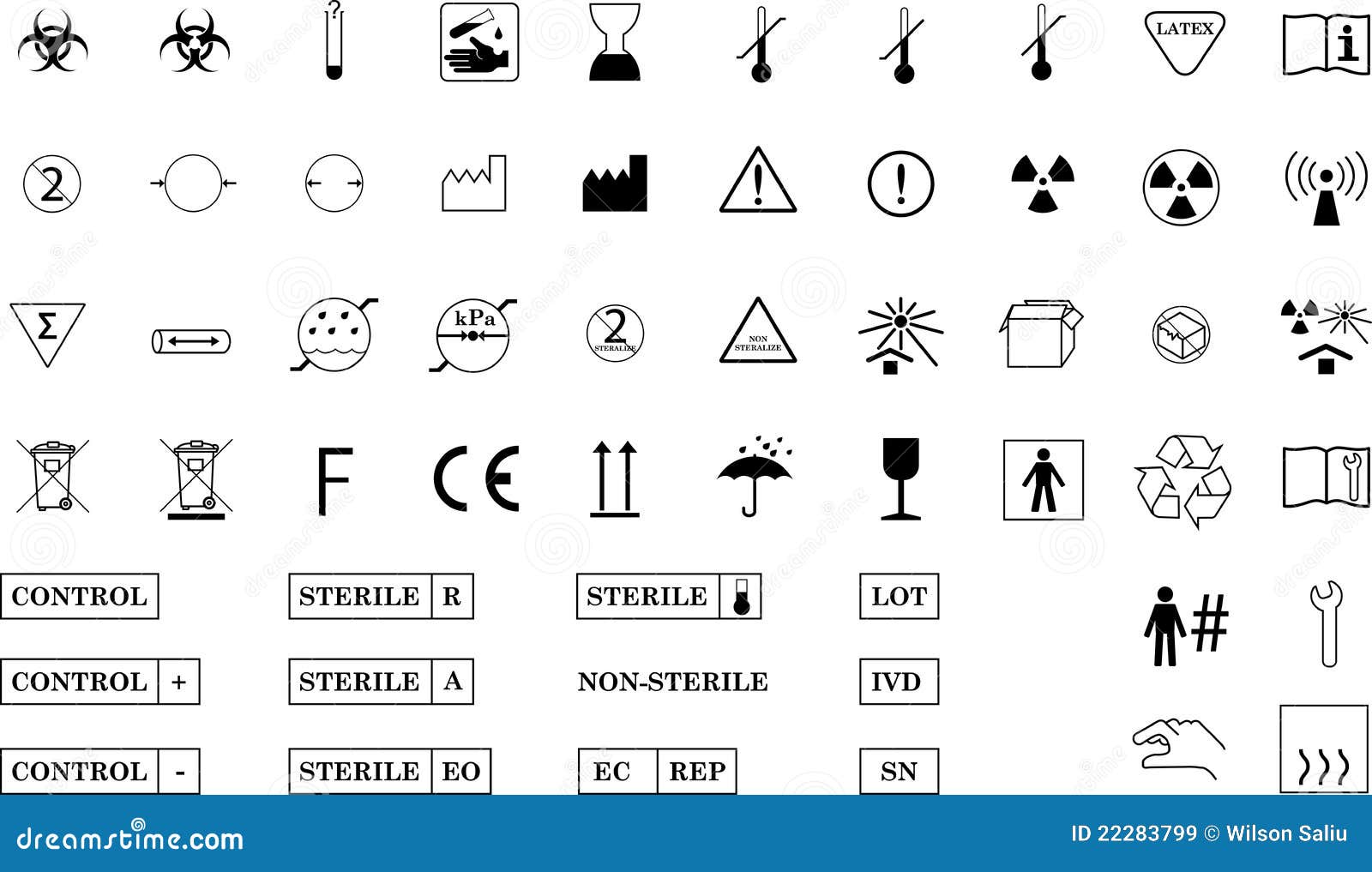
Iso Medical Device Label Standards . this document is applicable to symbols used in a broad spectrum of medical devices, that are available. this document includes the generally applicable requirements for identification and labels on a medical. Indicates a medical device that should not be used if the package has been damaged or opened. specifically, this document provides guidance on the content of the label, instructions for use, and information. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to.
from ectcolllefi.cf
specifically, this document provides guidance on the content of the label, instructions for use, and information. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this document is applicable to symbols used in a broad spectrum of medical devices, that are available. Indicates a medical device that should not be used if the package has been damaged or opened. this document includes the generally applicable requirements for identification and labels on a medical.
Iso 15223 1 2012 Medical Devices symbols to be Used with Medical device labels Labelling and
Iso Medical Device Label Standards this document includes the generally applicable requirements for identification and labels on a medical. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. specifically, this document provides guidance on the content of the label, instructions for use, and information. this document is applicable to symbols used in a broad spectrum of medical devices, that are available. Indicates a medical device that should not be used if the package has been damaged or opened. this document includes the generally applicable requirements for identification and labels on a medical.
From fra.animalia-life.club
Iso 15223 1 Symboles Iso Medical Device Label Standards this document is applicable to symbols used in a broad spectrum of medical devices, that are available. Indicates a medical device that should not be used if the package has been damaged or opened. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. specifically, this document provides guidance on. Iso Medical Device Label Standards.
From mungfali.com
Medical Device Labeling Symbols Iso Medical Device Label Standards this document includes the generally applicable requirements for identification and labels on a medical. this document is applicable to symbols used in a broad spectrum of medical devices, that are available. Indicates a medical device that should not be used if the package has been damaged or opened. specifically, this document provides guidance on the content of. Iso Medical Device Label Standards.
From medicaldeviceacademy.com
FDA medical device labeling regulations Archives Medical Device Academy Iso Medical Device Label Standards this document is applicable to symbols used in a broad spectrum of medical devices, that are available. specifically, this document provides guidance on the content of the label, instructions for use, and information. this document includes the generally applicable requirements for identification and labels on a medical. Indicates a medical device that should not be used if. Iso Medical Device Label Standards.
From old.sermitsiaq.ag
Medical Device Label Template Iso Medical Device Label Standards this document is applicable to symbols used in a broad spectrum of medical devices, that are available. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this document includes the generally applicable requirements for identification and labels on a medical. specifically, this document provides guidance on the content. Iso Medical Device Label Standards.
From printabletemplate.conaresvirtual.edu.sv
Medical Device Label Template Iso Medical Device Label Standards this document is applicable to symbols used in a broad spectrum of medical devices, that are available. this document includes the generally applicable requirements for identification and labels on a medical. specifically, this document provides guidance on the content of the label, instructions for use, and information. Indicates a medical device that should not be used if. Iso Medical Device Label Standards.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Iso Medical Device Label Standards this document is applicable to symbols used in a broad spectrum of medical devices, that are available. specifically, this document provides guidance on the content of the label, instructions for use, and information. this document includes the generally applicable requirements for identification and labels on a medical. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements. Iso Medical Device Label Standards.
From ambitiousmares.blogspot.com
34 Medical Device Label Symbols Labels Design Ideas 2020 Iso Medical Device Label Standards specifically, this document provides guidance on the content of the label, instructions for use, and information. Indicates a medical device that should not be used if the package has been damaged or opened. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this document is applicable to symbols used. Iso Medical Device Label Standards.
From www.vrogue.co
Medical Device Labeling Requirements In The Philippin vrogue.co Iso Medical Device Label Standards this document includes the generally applicable requirements for identification and labels on a medical. specifically, this document provides guidance on the content of the label, instructions for use, and information. Indicates a medical device that should not be used if the package has been damaged or opened. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that. Iso Medical Device Label Standards.
From mungfali.com
ISO Standard Label Symbols Iso Medical Device Label Standards Indicates a medical device that should not be used if the package has been damaged or opened. this document is applicable to symbols used in a broad spectrum of medical devices, that are available. specifically, this document provides guidance on the content of the label, instructions for use, and information. this document includes the generally applicable requirements. Iso Medical Device Label Standards.
From mavink.com
Medical Device Labeling Symbols Iso Medical Device Label Standards this document includes the generally applicable requirements for identification and labels on a medical. Indicates a medical device that should not be used if the package has been damaged or opened. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this document is applicable to symbols used in a. Iso Medical Device Label Standards.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Iso Medical Device Label Standards this document includes the generally applicable requirements for identification and labels on a medical. Indicates a medical device that should not be used if the package has been damaged or opened. this document is applicable to symbols used in a broad spectrum of medical devices, that are available. specifically, this document provides guidance on the content of. Iso Medical Device Label Standards.
From www.tapecon.com
What Information Should You Include on Your Medical Device Label? Iso Medical Device Label Standards the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this document includes the generally applicable requirements for identification and labels on a medical. Indicates a medical device that should not be used if the package has been damaged or opened. specifically, this document provides guidance on the content of. Iso Medical Device Label Standards.
From texaslabelprinters.com
Medical Device Label Printers UDI Label Printers Iso Medical Device Label Standards the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. Indicates a medical device that should not be used if the package has been damaged or opened. specifically, this document provides guidance on the content of the label, instructions for use, and information. this document includes the generally applicable requirements. Iso Medical Device Label Standards.
From kvalito.ch
Risk Management for Medical Devices ISO 149712019 Kvalito Consulting Group Iso Medical Device Label Standards specifically, this document provides guidance on the content of the label, instructions for use, and information. this document is applicable to symbols used in a broad spectrum of medical devices, that are available. Indicates a medical device that should not be used if the package has been damaged or opened. this document includes the generally applicable requirements. Iso Medical Device Label Standards.
From clin-r.com
Labels for Medical Devices Clin R Iso Medical Device Label Standards this document includes the generally applicable requirements for identification and labels on a medical. this document is applicable to symbols used in a broad spectrum of medical devices, that are available. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. specifically, this document provides guidance on the content. Iso Medical Device Label Standards.
From www.vrogue.co
Iso 13485 Regulatory Requirements On Medical Devices vrogue.co Iso Medical Device Label Standards Indicates a medical device that should not be used if the package has been damaged or opened. this document is applicable to symbols used in a broad spectrum of medical devices, that are available. specifically, this document provides guidance on the content of the label, instructions for use, and information. this document includes the generally applicable requirements. Iso Medical Device Label Standards.
From lanetasuper.weebly.com
Iso 13485 medical devices Iso Medical Device Label Standards this document is applicable to symbols used in a broad spectrum of medical devices, that are available. this document includes the generally applicable requirements for identification and labels on a medical. specifically, this document provides guidance on the content of the label, instructions for use, and information. Indicates a medical device that should not be used if. Iso Medical Device Label Standards.
From clin-r.com
Labels for Medical Devices Clin R Iso Medical Device Label Standards this document includes the generally applicable requirements for identification and labels on a medical. specifically, this document provides guidance on the content of the label, instructions for use, and information. this document is applicable to symbols used in a broad spectrum of medical devices, that are available. Indicates a medical device that should not be used if. Iso Medical Device Label Standards.
From mungfali.com
ISO Medical Device Symbols Iso Medical Device Label Standards Indicates a medical device that should not be used if the package has been damaged or opened. this document includes the generally applicable requirements for identification and labels on a medical. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. specifically, this document provides guidance on the content of. Iso Medical Device Label Standards.
From exodjaqsq.blob.core.windows.net
Tga Medical Device Labeling Requirements at Tyrone Gaylord blog Iso Medical Device Label Standards the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this document includes the generally applicable requirements for identification and labels on a medical. specifically, this document provides guidance on the content of the label, instructions for use, and information. Indicates a medical device that should not be used if. Iso Medical Device Label Standards.
From www.aplyon.com
Medical Device Process Validation Procedure ISO 13485 and FDA QSR Compliant Iso Medical Device Label Standards the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this document is applicable to symbols used in a broad spectrum of medical devices, that are available. specifically, this document provides guidance on the content of the label, instructions for use, and information. Indicates a medical device that should not. Iso Medical Device Label Standards.
From knconsultingandservices.com
What is Labelling? Medical Device Consulting Company Iso Medical Device Label Standards this document includes the generally applicable requirements for identification and labels on a medical. Indicates a medical device that should not be used if the package has been damaged or opened. this document is applicable to symbols used in a broad spectrum of medical devices, that are available. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements. Iso Medical Device Label Standards.
From www.aplyon.com
Medical Device Labeling Procedure Bundle Iso Medical Device Label Standards this document is applicable to symbols used in a broad spectrum of medical devices, that are available. specifically, this document provides guidance on the content of the label, instructions for use, and information. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. Indicates a medical device that should not. Iso Medical Device Label Standards.
From www.greenlight.guru
Ultimate List of ISO Standards for Medical Devices Iso Medical Device Label Standards specifically, this document provides guidance on the content of the label, instructions for use, and information. this document includes the generally applicable requirements for identification and labels on a medical. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this document is applicable to symbols used in a. Iso Medical Device Label Standards.
From www.greenlight.guru
ISO Standards for Medical Devices Ultimate List & Overview Iso Medical Device Label Standards specifically, this document provides guidance on the content of the label, instructions for use, and information. this document is applicable to symbols used in a broad spectrum of medical devices, that are available. Indicates a medical device that should not be used if the package has been damaged or opened. the medical devices regulation 2017/745/eu (‘mdr’) has. Iso Medical Device Label Standards.
From mungfali.com
Medical Device Labeling Symbols Iso Medical Device Label Standards the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this document includes the generally applicable requirements for identification and labels on a medical. Indicates a medical device that should not be used if the package has been damaged or opened. specifically, this document provides guidance on the content of. Iso Medical Device Label Standards.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Iso Medical Device Label Standards Indicates a medical device that should not be used if the package has been damaged or opened. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this document is applicable to symbols used in a broad spectrum of medical devices, that are available. specifically, this document provides guidance on. Iso Medical Device Label Standards.
From www.alamy.com
Full set of medical device packaging symbols with warning information. Medicine package black Iso Medical Device Label Standards this document includes the generally applicable requirements for identification and labels on a medical. Indicates a medical device that should not be used if the package has been damaged or opened. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. specifically, this document provides guidance on the content of. Iso Medical Device Label Standards.
From www.linkedin.com
ISO 1522312021 Medical Device Symbols An indispensable part of labeling Iso Medical Device Label Standards the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this document is applicable to symbols used in a broad spectrum of medical devices, that are available. Indicates a medical device that should not be used if the package has been damaged or opened. this document includes the generally applicable. Iso Medical Device Label Standards.
From www.slideserve.com
PPT Medical Device Standards PowerPoint Presentation, free download ID2088592 Iso Medical Device Label Standards this document is applicable to symbols used in a broad spectrum of medical devices, that are available. Indicates a medical device that should not be used if the package has been damaged or opened. specifically, this document provides guidance on the content of the label, instructions for use, and information. this document includes the generally applicable requirements. Iso Medical Device Label Standards.
From globalstrategicsolutions.com
The Most Common Manufacturing ISO Standards for Medical Devices Global Strategic Solutions Iso Medical Device Label Standards Indicates a medical device that should not be used if the package has been damaged or opened. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this document includes the generally applicable requirements for identification and labels on a medical. specifically, this document provides guidance on the content of. Iso Medical Device Label Standards.
From www.tailoredlabel.com
UDI Label Requirements For FDA Medical Device Labels TLP Iso Medical Device Label Standards the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this document includes the generally applicable requirements for identification and labels on a medical. this document is applicable to symbols used in a broad spectrum of medical devices, that are available. specifically, this document provides guidance on the content. Iso Medical Device Label Standards.
From ectcolllefi.cf
Iso 15223 1 2012 Medical Devices symbols to be Used with Medical device labels Labelling and Iso Medical Device Label Standards the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. specifically, this document provides guidance on the content of the label, instructions for use, and information. Indicates a medical device that should not be used if the package has been damaged or opened. this document is applicable to symbols used. Iso Medical Device Label Standards.
From www.linkedin.com
Vivek Pokhare MSc. on LinkedIn Medical Device Labeling Understanding ISO 15223 Standard Iso Medical Device Label Standards this document includes the generally applicable requirements for identification and labels on a medical. specifically, this document provides guidance on the content of the label, instructions for use, and information. Indicates a medical device that should not be used if the package has been damaged or opened. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that. Iso Medical Device Label Standards.
From cliniexperts.com
ISO 13485 Medical Devices Certification Medical Device ISO Standards CliniExperts Iso Medical Device Label Standards this document includes the generally applicable requirements for identification and labels on a medical. this document is applicable to symbols used in a broad spectrum of medical devices, that are available. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. Indicates a medical device that should not be used. Iso Medical Device Label Standards.