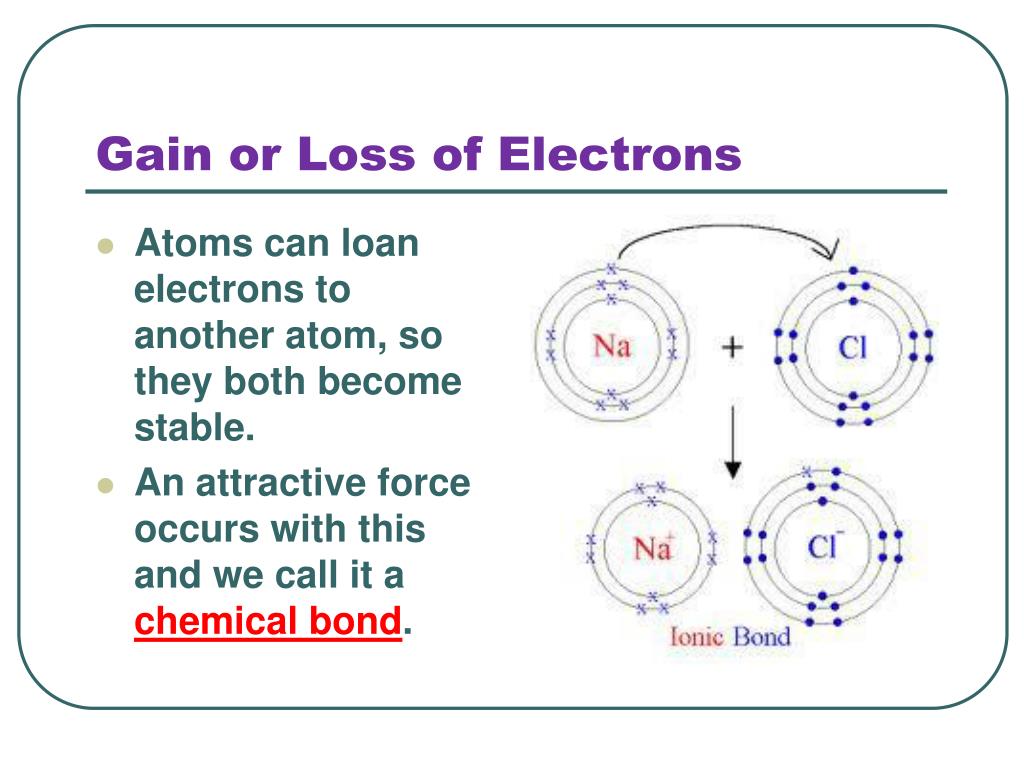
Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell . atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. the number of electrons in an element’s outer shell determines the number of electrons it will gain or lose to achieve a full outer shell. The ions formed are negative, because they have. A full valence shell is the most stable electron. group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. the octet rule states that atoms become especially stable when their valence shells gain a full complement of valence electrons. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron. but it explains why the number of electrons in a specific shell's full state is what it is, not why it's more stable than.
from www.slideserve.com
The ions formed are negative, because they have. group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. but it explains why the number of electrons in a specific shell's full state is what it is, not why it's more stable than. the octet rule states that atoms become especially stable when their valence shells gain a full complement of valence electrons. A full valence shell is the most stable electron. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. the number of electrons in an element’s outer shell determines the number of electrons it will gain or lose to achieve a full outer shell.
PPT Chapter 22 Chemical Bonds PowerPoint Presentation, free download ID5660961
Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell A full valence shell is the most stable electron. the number of electrons in an element’s outer shell determines the number of electrons it will gain or lose to achieve a full outer shell. the octet rule states that atoms become especially stable when their valence shells gain a full complement of valence electrons. but it explains why the number of electrons in a specific shell's full state is what it is, not why it's more stable than. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. A full valence shell is the most stable electron. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. A full valence shell is the most stable electron. The ions formed are negative, because they have.
From www.slideserve.com
PPT Chapter 22 Chemical Bonds PowerPoint Presentation, free download ID5660961 Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell A full valence shell is the most stable electron. A full valence shell is the most stable electron. the number of electrons in an element’s outer shell determines the number of electrons it will gain or lose to achieve a full outer shell. but it explains why the number of electrons in a specific shell's full state is. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron configuration, chemical data, and Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell but it explains why the number of electrons in a specific shell's full state is what it is, not why it's more stable than. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. group 18 elements (helium, neon, and argon are shown) have a full outer,. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From slideplayer.com
Unit 5 Electrons in Atoms ppt video online download Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. The ions formed are negative, because they have. the number of electrons in an element’s outer shell determines the number of electrons it will gain or lose to achieve a full outer shell. A full valence shell is the most stable electron.. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From dxottvosi.blob.core.windows.net
Is Bromine Lose Or Gain Electrons at Sharon Rakes blog Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell but it explains why the number of electrons in a specific shell's full state is what it is, not why it's more stable than. the number of electrons in an element’s outer shell determines the number of electrons it will gain or lose to achieve a full outer shell. A full valence shell is the most stable electron.. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From www.youtube.com
Electron Configuration of Bromine, Br YouTube Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell the octet rule states that atoms become especially stable when their valence shells gain a full complement of valence electrons. The ions formed are negative, because they have. group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. group 18 elements (helium, neon, and argon are shown in figure 2) have. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Table of the Elements Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. but it explains why the number of electrons in a specific shell's full state is what it is, not why it's. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell but it explains why the number of electrons in a specific shell's full state is what it is, not why it's more stable than. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. the number of electrons in an element’s outer shell determines the number of electrons. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From www.slideserve.com
PPT Chemistry Unit 6 PowerPoint Presentation, free download ID6652385 Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell the number of electrons in an element’s outer shell determines the number of electrons it will gain or lose to achieve a full outer shell. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. A full valence shell is the most stable electron. The ions formed are. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Forces Bind Atoms Together? Owlcation Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. A full valence shell is the most stable electron. The ions formed are negative, because they have. the octet rule states that atoms become especially stable when their valence shells gain a full complement of valence electrons. . Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From slideplayer.com
Periodic Table Of Elements ppt download Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell A full valence shell is the most stable electron. but it explains why the number of electrons in a specific shell's full state is what it is, not why it's more stable than. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. atoms tend to lose, gain,. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. the number of electrons in an element’s outer shell determines the number of electrons it will gain or lose to achieve a full outer shell. A full valence shell is the most stable electron. group 18 elements (helium, neon, and argon. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From slideplayer.com
Periodic Table Of Elements ppt download Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell the octet rule states that atoms become especially stable when their valence shells gain a full complement of valence electrons. A full valence shell is the most stable electron. The ions formed are negative, because they have. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. atoms. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From www.animalia-life.club
Electron Configuration For Bromine Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. The ions formed are negative, because they have. A full valence shell is the most stable electron. but it explains why the number of electrons in a specific shell's full state is what it is, not why it's more stable than. . Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From mungfali.com
Aufbau Diagram For Bromine Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. A full valence shell is the most stable electron. the number of electrons in an element’s outer shell determines the number of electrons it will gain or lose to achieve a full outer shell. The ions formed are. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From www.goodscience.com.au
Formation of Ions and Ionic Compounds Good Science Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. A full valence shell is the most stable electron. but it explains why the number of electrons in a specific shell's full state is what it is, not why it's more stable than. the number of electrons. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron configuration, and valence orbitals Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron. The ions formed are negative, because they have. the number of electrons in an element’s outer shell determines the number of electrons it will gain or lose to achieve a. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From www.breakingatom.com
Covalent Bonding Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell A full valence shell is the most stable electron. but it explains why the number of electrons in a specific shell's full state is what it is, not why it's more stable than. the octet rule states that atoms become especially stable when their valence shells gain a full complement of valence electrons. The ions formed are negative,. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From valenceelectrons.com
How to Find the Valence Electrons for Bromine (Br)? Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. the number of electrons in an element’s outer shell determines the number of electrons it will gain or lose to achieve a full outer shell. A full valence shell is the most stable electron. The ions formed are negative,. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From www.vrogue.co
Bromine Electron Configuration Br With Orbital Diagra vrogue.co Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell The ions formed are negative, because they have. group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. A full valence shell is the most stable electron. A full valence shell is the most stable electron. but it explains why the number of electrons in a specific shell's full state is what. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From chemistry.about.com
Atoms Diagrams Electron Configurations of Elements Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell A full valence shell is the most stable electron. but it explains why the number of electrons in a specific shell's full state is what it is, not why it's more stable than. group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. the octet rule states that atoms become especially. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From www.animalia-life.club
Electron Configuration For Bromine Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. the octet rule states that atoms become especially stable when their valence shells gain a full complement of valence electrons. The ions formed are negative, because they have. A full valence shell is the most stable electron. atoms. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition and electron configuration of an atom of Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell The ions formed are negative, because they have. the octet rule states that atoms become especially stable when their valence shells gain a full complement of valence electrons. the number of electrons in an element’s outer shell determines the number of electrons it will gain or lose to achieve a full outer shell. A full valence shell is. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From www.slideserve.com
PPT Draw Bromine Electron Shell Configuration 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5 Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell A full valence shell is the most stable electron. the number of electrons in an element’s outer shell determines the number of electrons it will gain or lose to achieve a full outer shell. group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. The ions formed are negative, because they have.. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From ar.inspiredpencil.com
Orbital Diagram Of Bromine Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron. The ions formed are negative, because they have. the octet rule states that atoms become especially stable when their valence shells gain a full complement of valence electrons. the. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell the octet rule states that atoms become especially stable when their valence shells gain a full complement of valence electrons. A full valence shell is the most stable electron. The ions formed are negative, because they have. group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. atoms tend to lose,. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From www.istockphoto.com
Br Bromine Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell A full valence shell is the most stable electron. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. but it explains why the number of electrons in a specific shell's full state is what it is, not why it's more stable than. A full valence shell is the. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From stewart-switch.com
Electron Dot Diagram For Bromine Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. the number of electrons in an element’s outer shell determines the number of electrons it will gain or lose to achieve. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From www.youtube.com
The bromine atom possesses 35 electrons. It contains 6 electrons in 2p orbital, 6 electrons in Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. the number of electrons in an element’s outer shell determines the number of electrons it will gain or lose to achieve a full outer shell. the octet rule states that atoms become especially stable when their valence. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From dxottvosi.blob.core.windows.net
Is Bromine Lose Or Gain Electrons at Sharon Rakes blog Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell The ions formed are negative, because they have. A full valence shell is the most stable electron. but it explains why the number of electrons in a specific shell's full state is what it is, not why it's more stable than. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From www.coursehero.com
[Solved] Neutral bromine (Br2) can gain two electrons to the anion... Course Hero Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell A full valence shell is the most stable electron. the number of electrons in an element’s outer shell determines the number of electrons it will gain or lose to achieve a full outer shell. group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. the octet rule states that atoms become. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From exoedqopw.blob.core.windows.net
Ionization Energy Of Bromine Equation at David Taber blog Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell A full valence shell is the most stable electron. The ions formed are negative, because they have. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. group. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From www.numerade.com
SOLVED 0 / 1 pts Question 11 As per octet rule, bromine atom would most likely valence shell Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell but it explains why the number of electrons in a specific shell's full state is what it is, not why it's more stable than. the octet rule states that atoms become especially stable when their valence shells gain a full complement of valence electrons. A full valence shell is the most stable electron. The ions formed are negative,. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From www.nagwa.com
Question Video Explaining the Reactions of an Element With Three Outer Shell Electrons Nagwa Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell the octet rule states that atoms become especially stable when their valence shells gain a full complement of valence electrons. but it explains why the number of electrons in a specific shell's full state is what it is, not why it's more stable than. atoms tend to lose, gain, or share some valance electrons, making bonds to. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From www.nagwa.com
Question Video Calculating the Number of Electrons That Must Be Lost for an Atom to Gain a Full Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. The ions formed are negative, because they have. A full valence shell is the most stable electron. group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. A full valence shell is. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. The ions formed are negative, because they have. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. the octet rule states that atoms become especially stable when their valence shells gain. Will Bromine Gain Or Lose Electrons To Get A Full Outer Shell.