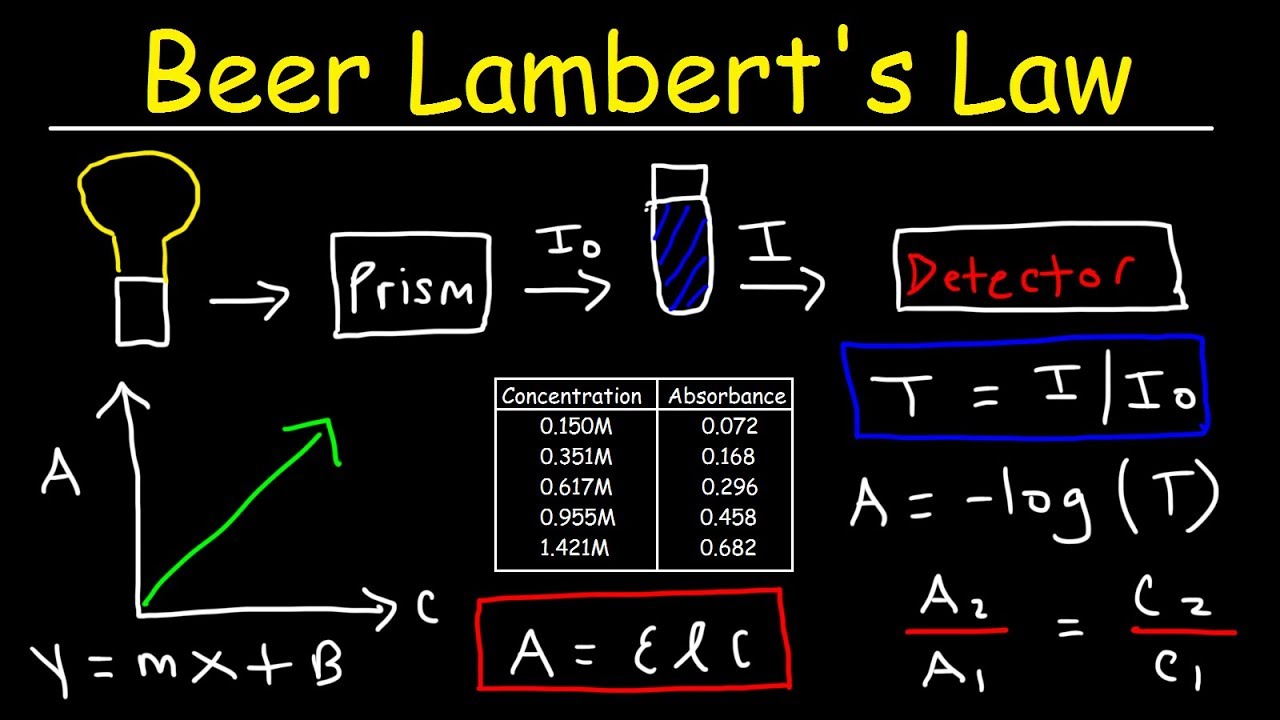
Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration . Set up the equation of the line to show the relation between absorbance and concentration. Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as.
from www.youtube.com
\[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Set up the equation of the line to show the relation between absorbance and concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant.
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry
Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. Set up the equation of the line to show the relation between absorbance and concentration. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as.
From www.youtube.com
Beer's Law Overview YouTube Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. Set up the equation of the line to show the relation between absorbance and concentration.. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Set up the equation of the line to show the relation between absorbance and concentration. Beer stated the transmittance of a solution is constant if the product of. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From www.youtube.com
Calculate concentration from UVVis absorbance using BeerLambert's law Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From www.youtube.com
Beer Lambert Law simplest explanation animation YouTube Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Set up the equation of the line to show the relation between absorbance and concentration. Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Set up the equation of the line to. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. Set up the equation of the line to show the relation between absorbance and concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as.. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From www.researchgate.net
Schematics demonstrating the original BeerLambert Law and Modified Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Set up the equation of the line to show the relation between absorbance and concentration. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer stated the transmittance of a solution is constant if the product of. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From www.slideserve.com
PPT Absorption and Scattering PowerPoint Presentation, free download Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Set up the equation of the line to show the relation between absorbance and concentration. Beer stated the transmittance of a solution is constant if the product of. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Set up the equation of the line to. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From www.slideserve.com
PPT Determining the Concentration of a Solution Beer’s Law Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Set up the equation of the line to show the relation between absorbance and concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer stated the transmittance of a solution is constant if the product of. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From mungfali.com
UV Spectroscopy Beer Lambert Law Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to show the relation between absorbance and concentration. Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant.. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From slidetodoc.com
Beers Lambert Law and Standard Curves of concentrations Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Set up the equation of the line to. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From www.chegg.com
Solved explain relation of BeerLambert Law with absorption Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Set up the equation of the line to show the relation between absorbance and concentration. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer stated the transmittance of a solution is constant if the product of. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Set up the equation of the line to show the relation between absorbance and concentration. Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as.. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From www.youtube.com
Absorption law (ii) Beer’s Law UV and Visible Spectroscopy YouTube Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Set up the equation of the line to. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Set up the equation of the line to show the relation between absorbance and concentration. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer stated the transmittance of a solution is constant if the product of. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From www.numerade.com
Question 20 1 pts The BeerLambert law relates the absorbance A of a Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to show the relation between absorbance and concentration.. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From www.youtube.com
Absorption spectroscopy (BeerLambert law) presented by PhD Emil Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Set up the equation of the line to show the relation between absorbance and concentration. Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. Set up the equation of the line to show the relation between absorbance and concentration. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From sciencenotes.org
Beer's Law Equation and Example Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Set up the equation of the line to show the relation between absorbance and concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From www.slideserve.com
PPT Protein Concentration Determination PowerPoint Presentation ID Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Set up the equation of the line to show the relation between absorbance and concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From www.thoughtco.com
Beer's Law Definition and Equation Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Set up the equation of the line to show the relation between absorbance and concentration. Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From stock.adobe.com
Vector scheme of Beer Lambert law. Cuvette with the blue liquid sample Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Set up the equation of the line to show the relation between absorbance and concentration. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer stated the transmittance of a solution is constant if the product of. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From www.scribd.com
BeerLambert Law Concentration (G/ML) x10 Absorbance PDF Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Set up the equation of the line to show the relation between absorbance and concentration. Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as.. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From webapi.bu.edu
💄 Beer lambert law absorption. Beer’s Law. 20221109 Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. Set up the equation of the line to show the relation between absorbance and concentration. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From www.youtube.com
Lambert Beer Law Absorption laws spectroscopy spectroscopy organic Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to show the relation between absorbance and concentration. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Beer stated the transmittance of a solution is constant if the product of. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From allaboutchemistry123.blogspot.com
Fundamental laws of absorption Beer Lambert law , definition and Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to show the relation between absorbance and concentration. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Beer stated the transmittance of a solution is constant if the product of. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Set up the equation of the line to show the relation between absorbance and concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer stated the transmittance of a solution is constant if the product of. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From www.youtube.com
Beer Lambert law derivation and usage YouTube Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Set up the equation of the line to show the relation between absorbance and concentration. Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as.. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From slidetodoc.com
Part 2 9 Electronic Transitions Outline Absorption spectroscopy Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to show the relation between absorbance and concentration.. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From www.researchgate.net
Schematics demonstrating the original BeerLambert Law and Modified Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Beer stated the transmittance of a solution is constant if the product of the path length and concentration are constant. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to show the relation between absorbance and concentration.. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.
From byjus.com
State and explain Beer Lambert Law. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Set up the equation of the line to show the relation between absorbance and concentration. Beer stated the transmittance of a solution is constant if the product of. Beer Lambert Law Explains The Absorption Behavior Of Solutions With Concentration.