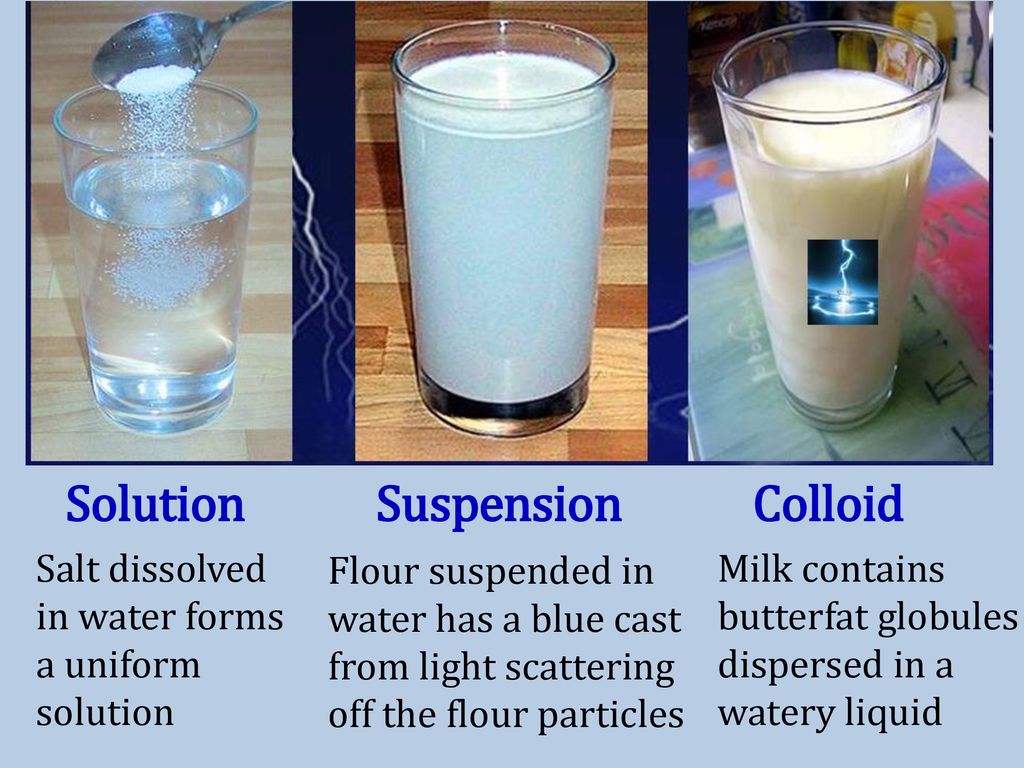
Suspension And Colloid Similarities . Solutions, colloids, and suspensions differ. colloids often appear opaque or translucent, while suspensions are typically opaque and heterogeneous. Colloid particles are much smaller than suspension. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. Solutions, colloids, and suspensions are all types of mixtures. the main difference between colloid and suspension lies in the size of particles. List and explain several technological applications of. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. describe the composition and properties of colloidal dispersions.
from slideplayer.com
Colloid particles are much smaller than suspension. Solutions, colloids, and suspensions are all types of mixtures. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. describe the composition and properties of colloidal dispersions. Solutions, colloids, and suspensions differ. the main difference between colloid and suspension lies in the size of particles. colloids often appear opaque or translucent, while suspensions are typically opaque and heterogeneous. List and explain several technological applications of. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and.
Solutions, Colloids, and Suspensions ppt download
Suspension And Colloid Similarities a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. Colloid particles are much smaller than suspension. describe the composition and properties of colloidal dispersions. the main difference between colloid and suspension lies in the size of particles. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. Solutions, colloids, and suspensions are all types of mixtures. colloids often appear opaque or translucent, while suspensions are typically opaque and heterogeneous. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. List and explain several technological applications of. Solutions, colloids, and suspensions differ.
From askanydifference.com
Colloid vs Suspension Difference and Comparison Suspension And Colloid Similarities the main difference between colloid and suspension lies in the size of particles. Solutions, colloids, and suspensions are all types of mixtures. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Solutions, colloids, and suspensions differ. describe the composition and properties of colloidal dispersions. colloids often. Suspension And Colloid Similarities.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Suspension And Colloid Similarities List and explain several technological applications of. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. describe the composition and properties of colloidal dispersions. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Colloid particles are much. Suspension And Colloid Similarities.
From brainly.in
How are colloids and suspensions different from solutions ? Brainly.in Suspension And Colloid Similarities Colloid particles are much smaller than suspension. colloids often appear opaque or translucent, while suspensions are typically opaque and heterogeneous. List and explain several technological applications of. Solutions, colloids, and suspensions are all types of mixtures. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. a colloid. Suspension And Colloid Similarities.
From www.numerade.com
SOLVED B. Suspension colloids, suspensions, and coarse mixture. Fill Suspension And Colloid Similarities Solutions, colloids, and suspensions are all types of mixtures. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. List and explain several technological applications of. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. suspensions and colloids. Suspension And Colloid Similarities.
From printableciascunaag.z21.web.core.windows.net
Solutions Colloids And Suspensions Worksheet Suspension And Colloid Similarities a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Colloid particles are much smaller than suspension. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. Solutions, colloids, and suspensions differ. a colloid is a mixture. Suspension And Colloid Similarities.
From www.slideserve.com
PPT SOLUTION CHEMISTRY PowerPoint Presentation, free download ID Suspension And Colloid Similarities describe the composition and properties of colloidal dispersions. colloids often appear opaque or translucent, while suspensions are typically opaque and heterogeneous. Colloid particles are much smaller than suspension. the main difference between colloid and suspension lies in the size of particles. List and explain several technological applications of. Solutions, colloids, and suspensions are all types of mixtures.. Suspension And Colloid Similarities.
From byjus.com
Suspensions (Chemistry) Definition, Properties, Examples with Videos Suspension And Colloid Similarities Solutions, colloids, and suspensions differ. colloids often appear opaque or translucent, while suspensions are typically opaque and heterogeneous. describe the composition and properties of colloidal dispersions. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. a colloid is a heterogeneous mixture in which the dispersed particles. Suspension And Colloid Similarities.
From www.differencebetween.com
Difference Between Suspension and Colloid Compare the Difference Suspension And Colloid Similarities the main difference between colloid and suspension lies in the size of particles. colloids often appear opaque or translucent, while suspensions are typically opaque and heterogeneous. List and explain several technological applications of. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. suspensions and colloids are. Suspension And Colloid Similarities.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True Suspension And Colloid Similarities Solutions, colloids, and suspensions are all types of mixtures. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. the main difference between colloid and suspension lies in the size of particles. a colloid is a mixture in which one of the soluble or insoluble particles. Suspension And Colloid Similarities.
From www.geeksforgeeks.org
Suspension(Chemistry) Definition, Properties, Examples, and FAQs Suspension And Colloid Similarities List and explain several technological applications of. Colloid particles are much smaller than suspension. describe the composition and properties of colloidal dispersions. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. colloids often appear opaque or translucent, while suspensions are typically opaque and heterogeneous. Solutions, colloids, and. Suspension And Colloid Similarities.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Suspension And Colloid Similarities a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. describe the composition and properties of colloidal dispersions. colloids often appear opaque or translucent, while suspensions are typically opaque and heterogeneous. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate. Suspension And Colloid Similarities.
From www.youtube.com
SCIENCE 6 SUSPENSION AND COLLOID YouTube Suspension And Colloid Similarities suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. colloids often appear opaque or translucent, while suspensions are typically opaque and heterogeneous. List and explain several. Suspension And Colloid Similarities.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz Suspension And Colloid Similarities Solutions, colloids, and suspensions differ. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. List and explain several technological applications of. a colloid is a mixture. Suspension And Colloid Similarities.
From www.studypool.com
SOLUTION Comparison of solution colloid and suspension and their Suspension And Colloid Similarities a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Solutions, colloids, and suspensions differ. Colloid particles are much smaller than suspension. Solutions, colloids, and suspensions are all types of mixtures. colloids often appear opaque or translucent, while suspensions are typically opaque and heterogeneous. the main difference between. Suspension And Colloid Similarities.
From www.studypool.com
SOLUTION Comparison of solution colloid and suspension and their Suspension And Colloid Similarities a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. colloids often appear opaque or translucent, while suspensions are typically opaque and heterogeneous. describe the composition and properties of colloidal dispersions. Solutions, colloids, and suspensions are all types of mixtures. the main difference between colloid and suspension. Suspension And Colloid Similarities.
From pediaa.com
Difference Between Colloid and Suspension Definition, Properties Suspension And Colloid Similarities suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. the main difference between colloid and suspension lies in the size of particles. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. List and explain several. Suspension And Colloid Similarities.
From askanydifference.com
Colloid vs Suspension Difference and Comparison Suspension And Colloid Similarities describe the composition and properties of colloidal dispersions. colloids often appear opaque or translucent, while suspensions are typically opaque and heterogeneous. List and explain several technological applications of. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. the main difference between colloid and suspension lies in. Suspension And Colloid Similarities.
From www.studypool.com
SOLUTION Comparison of solution suspension and colloid Studypool Suspension And Colloid Similarities colloids often appear opaque or translucent, while suspensions are typically opaque and heterogeneous. Solutions, colloids, and suspensions are all types of mixtures. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. describe the composition and properties of colloidal dispersions. Colloid particles are much smaller than suspension. List. Suspension And Colloid Similarities.
From dxolbrjjh.blob.core.windows.net
Suspension Colloid Define at Howard Barber blog Suspension And Colloid Similarities Colloid particles are much smaller than suspension. the main difference between colloid and suspension lies in the size of particles. colloids often appear opaque or translucent, while suspensions are typically opaque and heterogeneous. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. List and explain several technological. Suspension And Colloid Similarities.
From www.studypool.com
SOLUTION Comparison of solution suspension and colloid Studypool Suspension And Colloid Similarities the main difference between colloid and suspension lies in the size of particles. colloids often appear opaque or translucent, while suspensions are typically opaque and heterogeneous. List and explain several technological applications of. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Solutions, colloids, and suspensions differ.. Suspension And Colloid Similarities.
From byjus.com
Suspensions & Colloids Difference Between Colloid & SuspensionByju's Suspension And Colloid Similarities Solutions, colloids, and suspensions are all types of mixtures. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. List and explain several technological applications of. colloids often appear opaque or translucent, while suspensions are typically opaque and heterogeneous. describe the composition and properties of colloidal. Suspension And Colloid Similarities.
From entertosuccess.blogspot.com
Enter To Success Comparison of Solution, Suspension, and Colloids Suspension And Colloid Similarities List and explain several technological applications of. Solutions, colloids, and suspensions are all types of mixtures. describe the composition and properties of colloidal dispersions. colloids often appear opaque or translucent, while suspensions are typically opaque and heterogeneous. Colloid particles are much smaller than suspension. suspensions and colloids are two common types of mixtures whose properties are in. Suspension And Colloid Similarities.
From www.slideserve.com
PPT 2 PowerPoint Presentation, free download ID6415741 Suspension And Colloid Similarities List and explain several technological applications of. colloids often appear opaque or translucent, while suspensions are typically opaque and heterogeneous. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. Solutions, colloids, and suspensions are all types of mixtures. a colloid is a mixture in which. Suspension And Colloid Similarities.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Suspension And Colloid Similarities Solutions, colloids, and suspensions are all types of mixtures. the main difference between colloid and suspension lies in the size of particles. List and explain several technological applications of. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. describe the composition and properties of colloidal dispersions. Colloid. Suspension And Colloid Similarities.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Suspension And Colloid Similarities List and explain several technological applications of. the main difference between colloid and suspension lies in the size of particles. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. Colloid particles are much smaller than suspension. describe the composition and properties of colloidal dispersions. Solutions,. Suspension And Colloid Similarities.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download Suspension And Colloid Similarities suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. Solutions, colloids, and suspensions differ. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. colloids often appear opaque or translucent, while suspensions are typically opaque and. Suspension And Colloid Similarities.
From www.differencebetween.com
Difference Between Colloid and Emulsion Compare the Difference Suspension And Colloid Similarities Colloid particles are much smaller than suspension. List and explain several technological applications of. colloids often appear opaque or translucent, while suspensions are typically opaque and heterogeneous. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Solutions, colloids, and suspensions are all types of mixtures. describe the. Suspension And Colloid Similarities.
From www.differencebetween.net
Difference Between Colloid and Suspension Difference Between Suspension And Colloid Similarities List and explain several technological applications of. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. the main difference between colloid and suspension lies in the size of particles. Solutions, colloids, and suspensions differ. colloids often appear opaque or translucent, while suspensions are typically opaque. Suspension And Colloid Similarities.
From www.studypool.com
SOLUTION Comparison of solution suspension and colloid Studypool Suspension And Colloid Similarities suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. the main difference between colloid and suspension lies in the size of particles. colloids often appear. Suspension And Colloid Similarities.
From www.studypool.com
SOLUTION Comparison of Properties of Solutions, Suspension and Suspension And Colloid Similarities a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Solutions, colloids, and suspensions are all types of mixtures. Colloid particles are much smaller than suspension. Solutions, colloids, and suspensions. Suspension And Colloid Similarities.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID2182578 Suspension And Colloid Similarities List and explain several technological applications of. Colloid particles are much smaller than suspension. the main difference between colloid and suspension lies in the size of particles. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. a colloid is a mixture in which one of the soluble. Suspension And Colloid Similarities.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of Suspension And Colloid Similarities Colloid particles are much smaller than suspension. the main difference between colloid and suspension lies in the size of particles. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. Solutions, colloids, and suspensions differ. colloids often appear opaque or translucent, while suspensions are typically opaque and heterogeneous.. Suspension And Colloid Similarities.
From www.slideserve.com
PPT Solution, Suspensions and Colloids PowerPoint Presentation, free Suspension And Colloid Similarities a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. the main difference between colloid and suspension lies in the size of particles. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. suspensions and colloids are two. Suspension And Colloid Similarities.
From www.pinterest.com
mixture suspension colloid Google Search Chemistry worksheets Suspension And Colloid Similarities a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Colloid particles are much smaller than suspension. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. describe the composition and properties of colloidal dispersions. Solutions, colloids, and suspensions. Suspension And Colloid Similarities.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Suspension And Colloid Similarities Solutions, colloids, and suspensions differ. the main difference between colloid and suspension lies in the size of particles. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. Solutions, colloids, and suspensions are all types of mixtures. describe the composition and properties of colloidal dispersions. List. Suspension And Colloid Similarities.