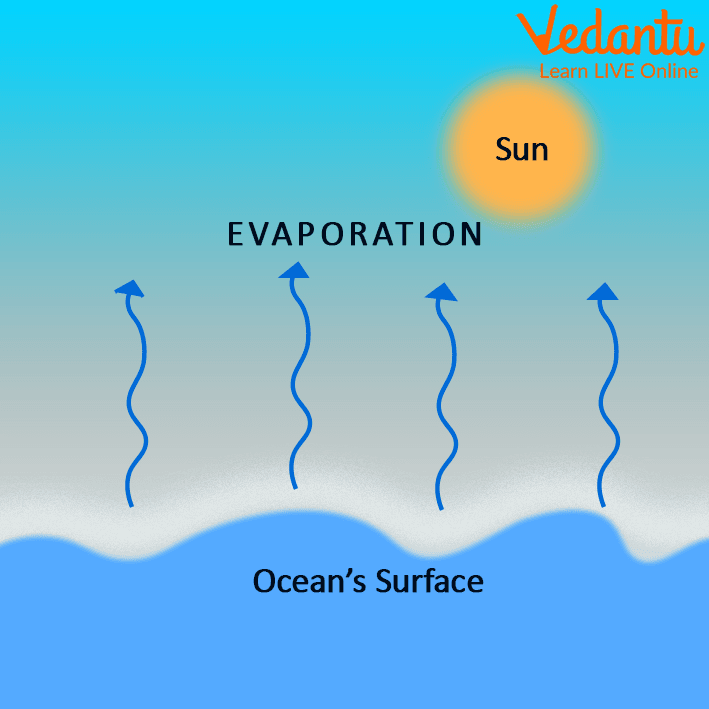
Evaporation Leaves Surfaces Cooler Because . The evaporation process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. In the case of evaporation it is highly relevant that the space available for the molecules to move in contributes to the. Damp washing hung out to dry feels cold. But in modern times we know that evaporation cause cooling because the particles (atoms and molecules) present at the surface of the liquid surface absorb energy from their surroundings and transform it into vapour, which then causes the cooling effect. You feel cooler when you get out of a swimming pool. The underlying principle behind this is that in order to change its state, the matter must either gain or lose energy. Evaporation, cooling, liquid bonds, kinetic energy
from www.vedantu.com
You feel cooler when you get out of a swimming pool. In the case of evaporation it is highly relevant that the space available for the molecules to move in contributes to the. The evaporation process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. Evaporation, cooling, liquid bonds, kinetic energy The underlying principle behind this is that in order to change its state, the matter must either gain or lose energy. But in modern times we know that evaporation cause cooling because the particles (atoms and molecules) present at the surface of the liquid surface absorb energy from their surroundings and transform it into vapour, which then causes the cooling effect. Damp washing hung out to dry feels cold.
Evaporation Learn Important Terms and Concepts
Evaporation Leaves Surfaces Cooler Because The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. Evaporation, cooling, liquid bonds, kinetic energy You feel cooler when you get out of a swimming pool. The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. The underlying principle behind this is that in order to change its state, the matter must either gain or lose energy. But in modern times we know that evaporation cause cooling because the particles (atoms and molecules) present at the surface of the liquid surface absorb energy from their surroundings and transform it into vapour, which then causes the cooling effect. The evaporation process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. Damp washing hung out to dry feels cold. In the case of evaporation it is highly relevant that the space available for the molecules to move in contributes to the.
From slideplayer.com
Energy & Matter Energy is not the only thing that moves through the Evaporation Leaves Surfaces Cooler Because Damp washing hung out to dry feels cold. The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. The evaporation process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. But in modern times we know that evaporation cause cooling because the particles (atoms and molecules) present at. Evaporation Leaves Surfaces Cooler Because.
From slideplayer.com
Plant Systems pp , ppt download Evaporation Leaves Surfaces Cooler Because The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. The underlying principle behind this is that in order to change its state, the matter must either gain or lose energy. Damp washing hung out to dry feels cold. The evaporation process leaves the remaining perspiration cooler, which in turn absorbs more heat. Evaporation Leaves Surfaces Cooler Because.
From stock.adobe.com
Belljar experiment on transpiration,transpiration is the evaporation of Evaporation Leaves Surfaces Cooler Because The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. Damp washing hung out to dry feels cold. In the case of evaporation it is highly relevant that the space available for the molecules to move in contributes to the. Evaporation, cooling, liquid bonds, kinetic energy The underlying principle behind this is that. Evaporation Leaves Surfaces Cooler Because.
From www.alamy.com
Water Cycle of Evaporation, Condensation, Precipitation to Collection Evaporation Leaves Surfaces Cooler Because Evaporation, cooling, liquid bonds, kinetic energy In the case of evaporation it is highly relevant that the space available for the molecules to move in contributes to the. The underlying principle behind this is that in order to change its state, the matter must either gain or lose energy. The evaporating process leaves the remaining perspiration cooler, which in turn. Evaporation Leaves Surfaces Cooler Because.
From www.youtube.com
How Indirect Evaporative Cooling Works YouTube Evaporation Leaves Surfaces Cooler Because Evaporation, cooling, liquid bonds, kinetic energy The evaporation process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. The underlying principle behind this is that in order to change its state, the matter must either gain or lose energy. Damp washing hung out to dry feels cold. But in modern times we know that evaporation. Evaporation Leaves Surfaces Cooler Because.
From stock.adobe.com
Vecteur Stock Leaf surface, transpiration. Expulsion of water vapor Evaporation Leaves Surfaces Cooler Because The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. The evaporation process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. You feel cooler when you get out of a swimming pool. The underlying principle behind this is that in order to change its state, the matter. Evaporation Leaves Surfaces Cooler Because.
From dokumen.tips
(PPTX) ESTIMATING EVAPORATION FROM WATER SURFACES DOKUMEN.TIPS Evaporation Leaves Surfaces Cooler Because But in modern times we know that evaporation cause cooling because the particles (atoms and molecules) present at the surface of the liquid surface absorb energy from their surroundings and transform it into vapour, which then causes the cooling effect. In the case of evaporation it is highly relevant that the space available for the molecules to move in contributes. Evaporation Leaves Surfaces Cooler Because.
From www.mrductcleaning.com.au
Why your evaporative cooler can cool down the air without a compressor Evaporation Leaves Surfaces Cooler Because But in modern times we know that evaporation cause cooling because the particles (atoms and molecules) present at the surface of the liquid surface absorb energy from their surroundings and transform it into vapour, which then causes the cooling effect. In the case of evaporation it is highly relevant that the space available for the molecules to move in contributes. Evaporation Leaves Surfaces Cooler Because.
From basc.pnnl.gov
Concept behind an evaporative cooler warm air is cooled as the air Evaporation Leaves Surfaces Cooler Because Evaporation, cooling, liquid bonds, kinetic energy The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. The underlying principle behind this is that in order to change its state, the matter must either gain or lose energy. But in modern times we know that evaporation cause cooling because the particles (atoms and molecules). Evaporation Leaves Surfaces Cooler Because.
From www.researchgate.net
Precrisis regimes of evaporation/boiling on surfaces, Ps = 400 Pa; Ps Evaporation Leaves Surfaces Cooler Because In the case of evaporation it is highly relevant that the space available for the molecules to move in contributes to the. The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. The evaporation process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. The underlying principle behind. Evaporation Leaves Surfaces Cooler Because.
From ar.inspiredpencil.com
Evaporation Evaporation Leaves Surfaces Cooler Because The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. In the case of evaporation it is highly relevant that the space available for the molecules to move in contributes to the. Damp washing hung out to dry feels cold. But in modern times we know that evaporation cause cooling because the particles. Evaporation Leaves Surfaces Cooler Because.
From sciencenotes.org
Evaporative Cooler How It Works and Examples Evaporation Leaves Surfaces Cooler Because The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. In the case of evaporation it is highly relevant that the space available for the molecules to move in contributes to the. Damp washing hung out to dry feels cold. The evaporation process leaves the remaining perspiration cooler, which in turn absorbs more. Evaporation Leaves Surfaces Cooler Because.
From www.teachoo.com
How does Evaporation cause cooling? Explain (with Examples) Teachoo Evaporation Leaves Surfaces Cooler Because The underlying principle behind this is that in order to change its state, the matter must either gain or lose energy. Evaporation, cooling, liquid bonds, kinetic energy In the case of evaporation it is highly relevant that the space available for the molecules to move in contributes to the. You feel cooler when you get out of a swimming pool.. Evaporation Leaves Surfaces Cooler Because.
From www.animalia-life.club
Evaporative Cooling Diagram Evaporation Leaves Surfaces Cooler Because Evaporation, cooling, liquid bonds, kinetic energy But in modern times we know that evaporation cause cooling because the particles (atoms and molecules) present at the surface of the liquid surface absorb energy from their surroundings and transform it into vapour, which then causes the cooling effect. In the case of evaporation it is highly relevant that the space available for. Evaporation Leaves Surfaces Cooler Because.
From www.alamy.com
Transpiration the leaf of a house plant close to a cool window shows Evaporation Leaves Surfaces Cooler Because The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. But in modern times we know that evaporation cause cooling because the particles (atoms and molecules) present at the surface of the liquid surface absorb energy from their surroundings and transform it into vapour, which then causes the cooling effect. The evaporation process. Evaporation Leaves Surfaces Cooler Because.
From www.labroots.com
Trees Can Help Cool the Environment Trending Evaporation Leaves Surfaces Cooler Because The underlying principle behind this is that in order to change its state, the matter must either gain or lose energy. But in modern times we know that evaporation cause cooling because the particles (atoms and molecules) present at the surface of the liquid surface absorb energy from their surroundings and transform it into vapour, which then causes the cooling. Evaporation Leaves Surfaces Cooler Because.
From www.dreamstime.com
Transpiration Vector Illustration. Labeled Educational Plant Water Evaporation Leaves Surfaces Cooler Because In the case of evaporation it is highly relevant that the space available for the molecules to move in contributes to the. The underlying principle behind this is that in order to change its state, the matter must either gain or lose energy. Damp washing hung out to dry feels cold. You feel cooler when you get out of a. Evaporation Leaves Surfaces Cooler Because.
From www.sewa.net.my
EVAPORATION AIR COOLER INDOOR & OUTDOOR SEWA Evaporation Leaves Surfaces Cooler Because The underlying principle behind this is that in order to change its state, the matter must either gain or lose energy. Evaporation, cooling, liquid bonds, kinetic energy You feel cooler when you get out of a swimming pool. But in modern times we know that evaporation cause cooling because the particles (atoms and molecules) present at the surface of the. Evaporation Leaves Surfaces Cooler Because.
From www.world-today-news.com
Evaporation of Water by Leaves, Here's the Definition and Benefits Evaporation Leaves Surfaces Cooler Because In the case of evaporation it is highly relevant that the space available for the molecules to move in contributes to the. But in modern times we know that evaporation cause cooling because the particles (atoms and molecules) present at the surface of the liquid surface absorb energy from their surroundings and transform it into vapour, which then causes the. Evaporation Leaves Surfaces Cooler Because.
From education.nationalgeographic.org
Evaporation Evaporation Leaves Surfaces Cooler Because The underlying principle behind this is that in order to change its state, the matter must either gain or lose energy. But in modern times we know that evaporation cause cooling because the particles (atoms and molecules) present at the surface of the liquid surface absorb energy from their surroundings and transform it into vapour, which then causes the cooling. Evaporation Leaves Surfaces Cooler Because.
From serc.carleton.edu
Evapotranspiration and Crop Water Use Evaporation Leaves Surfaces Cooler Because The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. The underlying principle behind this is that in order to change its state, the matter must either gain or lose energy. Evaporation, cooling, liquid bonds, kinetic energy But in modern times we know that evaporation cause cooling because the particles (atoms and molecules). Evaporation Leaves Surfaces Cooler Because.
From www.numerade.com
SOLVEDEvaporating sweat cools the body because evaporation is an Evaporation Leaves Surfaces Cooler Because Damp washing hung out to dry feels cold. But in modern times we know that evaporation cause cooling because the particles (atoms and molecules) present at the surface of the liquid surface absorb energy from their surroundings and transform it into vapour, which then causes the cooling effect. Evaporation, cooling, liquid bonds, kinetic energy You feel cooler when you get. Evaporation Leaves Surfaces Cooler Because.
From www.teachoo.com
Why is Evaporation called a surface phenomenon? Teachoo Evaporation Leaves Surfaces Cooler Because You feel cooler when you get out of a swimming pool. The underlying principle behind this is that in order to change its state, the matter must either gain or lose energy. Damp washing hung out to dry feels cold. In the case of evaporation it is highly relevant that the space available for the molecules to move in contributes. Evaporation Leaves Surfaces Cooler Because.
From www.youtube.com
Why evaporation is a cooling process? YouTube Evaporation Leaves Surfaces Cooler Because The underlying principle behind this is that in order to change its state, the matter must either gain or lose energy. You feel cooler when you get out of a swimming pool. Damp washing hung out to dry feels cold. The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. The evaporation process. Evaporation Leaves Surfaces Cooler Because.
From www.vedantu.com
Evaporation Learn Important Terms and Concepts Evaporation Leaves Surfaces Cooler Because The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. In the case of evaporation it is highly relevant that the space available for the molecules to move in contributes to the. The evaporation process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. Evaporation, cooling, liquid bonds,. Evaporation Leaves Surfaces Cooler Because.
From www.slideserve.com
PPT Evaporation PowerPoint Presentation, free download ID2598333 Evaporation Leaves Surfaces Cooler Because Evaporation, cooling, liquid bonds, kinetic energy The evaporation process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. You feel cooler when you get out of a swimming pool. But in modern times we know that evaporation cause cooling because the particles (atoms and molecules) present at the surface of the liquid surface absorb energy. Evaporation Leaves Surfaces Cooler Because.
From www.slideserve.com
PPT Entry of water into plants PowerPoint Presentation, free download Evaporation Leaves Surfaces Cooler Because Evaporation, cooling, liquid bonds, kinetic energy The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. Damp washing hung out to dry feels cold. But in modern times we know that evaporation cause cooling because the particles (atoms and molecules) present at the surface of the liquid surface absorb energy from their surroundings. Evaporation Leaves Surfaces Cooler Because.
From angel-owncreator.blogspot.com
Describe the Process of Transpiration and Evapotranspiration Evaporation Leaves Surfaces Cooler Because But in modern times we know that evaporation cause cooling because the particles (atoms and molecules) present at the surface of the liquid surface absorb energy from their surroundings and transform it into vapour, which then causes the cooling effect. The underlying principle behind this is that in order to change its state, the matter must either gain or lose. Evaporation Leaves Surfaces Cooler Because.
From www.goodfruit.com
evaporative cooling Good Fruit Grower Evaporation Leaves Surfaces Cooler Because In the case of evaporation it is highly relevant that the space available for the molecules to move in contributes to the. The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. The evaporation process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. You feel cooler when. Evaporation Leaves Surfaces Cooler Because.
From www.condair.com
What is an Evaporative Cooler Evaporation Leaves Surfaces Cooler Because The underlying principle behind this is that in order to change its state, the matter must either gain or lose energy. Damp washing hung out to dry feels cold. The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. But in modern times we know that evaporation cause cooling because the particles (atoms. Evaporation Leaves Surfaces Cooler Because.
From circuitlistbaroque99.z13.web.core.windows.net
How To Power Evaporation Cooler Evaporation Leaves Surfaces Cooler Because The underlying principle behind this is that in order to change its state, the matter must either gain or lose energy. The evaporation process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. Evaporation, cooling, liquid bonds, kinetic energy In the case of evaporation it is highly relevant that the space available for the molecules. Evaporation Leaves Surfaces Cooler Because.
From www.nagwa.com
Question Video Understanding the Process of Water Loss from the Evaporation Leaves Surfaces Cooler Because Evaporation, cooling, liquid bonds, kinetic energy The underlying principle behind this is that in order to change its state, the matter must either gain or lose energy. The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. You feel cooler when you get out of a swimming pool. But in modern times we. Evaporation Leaves Surfaces Cooler Because.
From www.shutterstock.com
Evaporation Vector Illustration Labeled Liquid Surface Stock Vector Evaporation Leaves Surfaces Cooler Because But in modern times we know that evaporation cause cooling because the particles (atoms and molecules) present at the surface of the liquid surface absorb energy from their surroundings and transform it into vapour, which then causes the cooling effect. The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. Damp washing hung. Evaporation Leaves Surfaces Cooler Because.
From www.teachoo.com
Evaporation Meaning and Factors affecting it Teachoo Evaporation Leaves Surfaces Cooler Because The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body. You feel cooler when you get out of a swimming pool. Damp washing hung out to dry feels cold. Evaporation, cooling, liquid bonds, kinetic energy The underlying principle behind this is that in order to change its state, the matter must either gain. Evaporation Leaves Surfaces Cooler Because.
From www.wur.nl
Synergy between a leaf on a tree and a cloud in the sky WUR Evaporation Leaves Surfaces Cooler Because The underlying principle behind this is that in order to change its state, the matter must either gain or lose energy. In the case of evaporation it is highly relevant that the space available for the molecules to move in contributes to the. Evaporation, cooling, liquid bonds, kinetic energy The evaporating process leaves the remaining perspiration cooler, which in turn. Evaporation Leaves Surfaces Cooler Because.