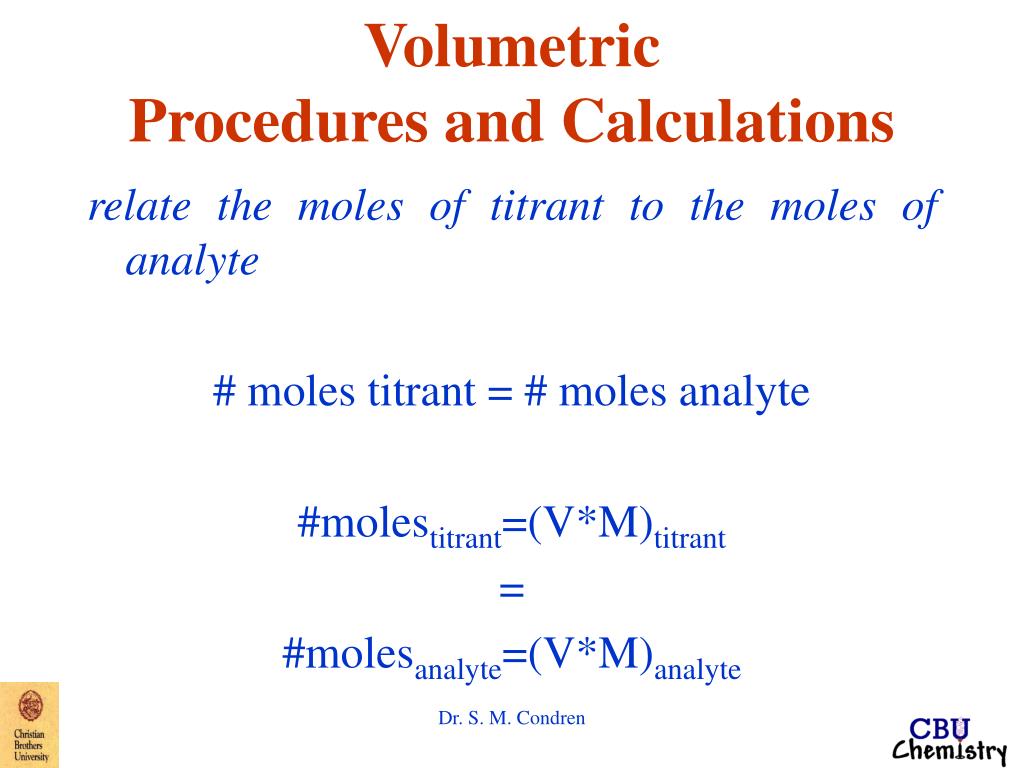
Correction Factor Volumetric Solutions . Volumetric solutions are standardised by the methods described below. Volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. The titer is defined as the quotient of the nominal concentration of a volumetric solution and the actual concentration. When a volumetric solution is to be. Cf = correction factor, calculated from the effective content of the volumetric. Correction factor is determined on the volumetric solution to be used in the monograph. 12 rows calculation of the mass fraction w (nacl) in mg/g solution: These can be connected directly to a titrator via. In the latter case, the correction factor of the dilute solution. Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods.
from www.slideserve.com
When a volumetric solution is to be. Volumetric solutions are standardised by the methods described below. Cf = correction factor, calculated from the effective content of the volumetric. Correction factor is determined on the volumetric solution to be used in the monograph. In the latter case, the correction factor of the dilute solution. Volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. The titer is defined as the quotient of the nominal concentration of a volumetric solution and the actual concentration. 12 rows calculation of the mass fraction w (nacl) in mg/g solution: These can be connected directly to a titrator via. Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods.
PPT Principles of Volumetric Analysis PowerPoint Presentation, free
Correction Factor Volumetric Solutions Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods. Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods. Cf = correction factor, calculated from the effective content of the volumetric. When a volumetric solution is to be. These can be connected directly to a titrator via. 12 rows calculation of the mass fraction w (nacl) in mg/g solution: Volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. In the latter case, the correction factor of the dilute solution. The titer is defined as the quotient of the nominal concentration of a volumetric solution and the actual concentration. Correction factor is determined on the volumetric solution to be used in the monograph. Volumetric solutions are standardised by the methods described below.
From www.researchgate.net
Variation of correction factor in terms of mean and mean ± standard Correction Factor Volumetric Solutions The titer is defined as the quotient of the nominal concentration of a volumetric solution and the actual concentration. In the latter case, the correction factor of the dilute solution. Correction factor is determined on the volumetric solution to be used in the monograph. Cf = correction factor, calculated from the effective content of the volumetric. These can be connected. Correction Factor Volumetric Solutions.
From www.slideserve.com
PPT Volumetric Analysis PowerPoint Presentation, free download ID Correction Factor Volumetric Solutions The titer is defined as the quotient of the nominal concentration of a volumetric solution and the actual concentration. Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods. Correction factor is determined on the volumetric solution to be used in the monograph. Volumetric solutions are standardised by the methods described below. In the latter case, the. Correction Factor Volumetric Solutions.
From www.narayannepal.com.np
Volumetric Analysis Class 12 Physical Chemistry Notes NEB Notes Correction Factor Volumetric Solutions In the latter case, the correction factor of the dilute solution. Cf = correction factor, calculated from the effective content of the volumetric. 12 rows calculation of the mass fraction w (nacl) in mg/g solution: These can be connected directly to a titrator via. Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods. Volumetric solutions are. Correction Factor Volumetric Solutions.
From www.youtube.com
Form three chemistry, Volumetric analysis session 3, Standard solution Correction Factor Volumetric Solutions When a volumetric solution is to be. 12 rows calculation of the mass fraction w (nacl) in mg/g solution: Volumetric solutions are standardised by the methods described below. Correction factor is determined on the volumetric solution to be used in the monograph. Cf = correction factor, calculated from the effective content of the volumetric. Volumetric analysis is a chemical analytical. Correction Factor Volumetric Solutions.
From www.slideserve.com
PPT Principles of Volumetric Analysis PowerPoint Presentation, free Correction Factor Volumetric Solutions Volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. These can be connected directly to a titrator via. The titer is defined as the quotient of the nominal concentration of a volumetric solution and the actual concentration. When a volumetric solution is to be. Volumetric solutions volumetric solutions are prepared according to the. Correction Factor Volumetric Solutions.
From www.youtube.com
Formula Of Factor Volumetric Solution Factor Formula YouTube Correction Factor Volumetric Solutions Volumetric solutions are standardised by the methods described below. Cf = correction factor, calculated from the effective content of the volumetric. 12 rows calculation of the mass fraction w (nacl) in mg/g solution: Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods. In the latter case, the correction factor of the dilute solution. The titer is. Correction Factor Volumetric Solutions.
From www.youtube.com
Calibration and Use of a Volumetric Pipet YouTube Correction Factor Volumetric Solutions Volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. Cf = correction factor, calculated from the effective content of the volumetric. The titer is defined as the quotient of the nominal concentration of a volumetric solution and the actual concentration. Correction factor is determined on the volumetric solution to be used in the. Correction Factor Volumetric Solutions.
From www.researchgate.net
The magnitude and phase of the correction factor for calculating the Correction Factor Volumetric Solutions Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods. Correction factor is determined on the volumetric solution to be used in the monograph. 12 rows calculation of the mass fraction w (nacl) in mg/g solution: When a volumetric solution is to be. Volumetric solutions are standardised by the methods described below. These can be connected directly. Correction Factor Volumetric Solutions.
From www.researchgate.net
Correction factor with various H\documentclass[12pt]{minimal Correction Factor Volumetric Solutions Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods. These can be connected directly to a titrator via. In the latter case, the correction factor of the dilute solution. The titer is defined as the quotient of the nominal concentration of a volumetric solution and the actual concentration. Volumetric analysis is a chemical analytical procedure based. Correction Factor Volumetric Solutions.
From www.numerade.com
SOLVED Calculate the viscosity correction factor for the volumetric Correction Factor Volumetric Solutions Cf = correction factor, calculated from the effective content of the volumetric. Correction factor is determined on the volumetric solution to be used in the monograph. In the latter case, the correction factor of the dilute solution. The titer is defined as the quotient of the nominal concentration of a volumetric solution and the actual concentration. Volumetric solutions volumetric solutions. Correction Factor Volumetric Solutions.
From www.studypool.com
SOLUTION Volume correction table Studypool Correction Factor Volumetric Solutions Volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. In the latter case, the correction factor of the dilute solution. Volumetric solutions are standardised by the methods described below. When a volumetric solution is to be. Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods. Cf = correction factor,. Correction Factor Volumetric Solutions.
From www.slideserve.com
PPT Principles of Volumetric Analysis PowerPoint Presentation, free Correction Factor Volumetric Solutions 12 rows calculation of the mass fraction w (nacl) in mg/g solution: When a volumetric solution is to be. Volumetric solutions are standardised by the methods described below. Correction factor is determined on the volumetric solution to be used in the monograph. Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods. The titer is defined as. Correction Factor Volumetric Solutions.
From tech-publish.com
90+ Volumetric Solutions Preparation And Standardization Techpublish Correction Factor Volumetric Solutions Cf = correction factor, calculated from the effective content of the volumetric. When a volumetric solution is to be. Volumetric solutions are standardised by the methods described below. Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods. 12 rows calculation of the mass fraction w (nacl) in mg/g solution: These can be connected directly to a. Correction Factor Volumetric Solutions.
From www.researchgate.net
Plot of totalactivity recoverycoefficient correction factor vs Correction Factor Volumetric Solutions 12 rows calculation of the mass fraction w (nacl) in mg/g solution: These can be connected directly to a titrator via. Volumetric solutions are standardised by the methods described below. When a volumetric solution is to be. The titer is defined as the quotient of the nominal concentration of a volumetric solution and the actual concentration. In the latter case,. Correction Factor Volumetric Solutions.
From www.researchgate.net
4. Determining the correction factor S for Example 63 At π / 1 Correction Factor Volumetric Solutions Correction factor is determined on the volumetric solution to be used in the monograph. Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods. The titer is defined as the quotient of the nominal concentration of a volumetric solution and the actual concentration. 12 rows calculation of the mass fraction w (nacl) in mg/g solution: Volumetric analysis. Correction Factor Volumetric Solutions.
From www.narayannepal.com.np
Volumetric Analysis Class 12 Physical Chemistry Notes NEB Notes Correction Factor Volumetric Solutions 12 rows calculation of the mass fraction w (nacl) in mg/g solution: Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods. The titer is defined as the quotient of the nominal concentration of a volumetric solution and the actual concentration. Cf = correction factor, calculated from the effective content of the volumetric. Volumetric solutions are standardised. Correction Factor Volumetric Solutions.
From www.researchgate.net
Volumetric pressure correction factor, C psm , for the QSonic 5 meter Correction Factor Volumetric Solutions Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods. These can be connected directly to a titrator via. Cf = correction factor, calculated from the effective content of the volumetric. Volumetric solutions are standardised by the methods described below. Correction factor is determined on the volumetric solution to be used in the monograph. When a volumetric. Correction Factor Volumetric Solutions.
From slideplayer.com
Chapter 0 The Analytical Process (Example) ppt download Correction Factor Volumetric Solutions Volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. When a volumetric solution is to be. These can be connected directly to a titrator via. Volumetric solutions are standardised by the methods described below. The titer is defined as the quotient of the nominal concentration of a volumetric solution and the actual concentration.. Correction Factor Volumetric Solutions.
From www.researchgate.net
A and B, Ion correction factor. Download Scientific Diagram Correction Factor Volumetric Solutions Volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. Volumetric solutions are standardised by the methods described below. These can be connected directly to a titrator via. 12 rows calculation of the mass fraction w (nacl) in mg/g solution: Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods. Correction. Correction Factor Volumetric Solutions.
From www.narayannepal.com.np
Volumetric Analysis Class 12 Physical Chemistry Notes NEB Notes Correction Factor Volumetric Solutions The titer is defined as the quotient of the nominal concentration of a volumetric solution and the actual concentration. These can be connected directly to a titrator via. Correction factor is determined on the volumetric solution to be used in the monograph. When a volumetric solution is to be. In the latter case, the correction factor of the dilute solution.. Correction Factor Volumetric Solutions.
From www.researchgate.net
Local liquid mass transfer coefficient k l (m s1 ) based on Equation Correction Factor Volumetric Solutions Correction factor is determined on the volumetric solution to be used in the monograph. Volumetric solutions are standardised by the methods described below. When a volumetric solution is to be. In the latter case, the correction factor of the dilute solution. The titer is defined as the quotient of the nominal concentration of a volumetric solution and the actual concentration.. Correction Factor Volumetric Solutions.
From www.narayannepal.com.np
Volumetric Analysis Class 12 Physical Chemistry Notes NEB Notes Correction Factor Volumetric Solutions Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods. These can be connected directly to a titrator via. Correction factor is determined on the volumetric solution to be used in the monograph. In the latter case, the correction factor of the dilute solution. Cf = correction factor, calculated from the effective content of the volumetric. When. Correction Factor Volumetric Solutions.
From www.youtube.com
Fluid Mechanics Lesson 12C Cunningham Correction Factor YouTube Correction Factor Volumetric Solutions These can be connected directly to a titrator via. Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods. The titer is defined as the quotient of the nominal concentration of a volumetric solution and the actual concentration. Volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. Cf = correction. Correction Factor Volumetric Solutions.
From www.researchgate.net
Volume correction factor, displayed as a function of experiment day Correction Factor Volumetric Solutions Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods. The titer is defined as the quotient of the nominal concentration of a volumetric solution and the actual concentration. These can be connected directly to a titrator via. In the latter case, the correction factor of the dilute solution. Volumetric solutions are standardised by the methods described. Correction Factor Volumetric Solutions.
From www.narayannepal.com.np
Volumetric Analysis Class 12 Physical Chemistry Notes NEB Notes Correction Factor Volumetric Solutions 12 rows calculation of the mass fraction w (nacl) in mg/g solution: Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods. The titer is defined as the quotient of the nominal concentration of a volumetric solution and the actual concentration. These can be connected directly to a titrator via. Volumetric analysis is a chemical analytical procedure. Correction Factor Volumetric Solutions.
From www.researchgate.net
4 The volume correction factor is applied to nodes that lie between δ Correction Factor Volumetric Solutions In the latter case, the correction factor of the dilute solution. These can be connected directly to a titrator via. 12 rows calculation of the mass fraction w (nacl) in mg/g solution: Cf = correction factor, calculated from the effective content of the volumetric. When a volumetric solution is to be. Volumetric solutions are standardised by the methods described below.. Correction Factor Volumetric Solutions.
From www.youtube.com
DSE04 Acids and Bases Volumetric Analysis calculation example (2 Correction Factor Volumetric Solutions 12 rows calculation of the mass fraction w (nacl) in mg/g solution: In the latter case, the correction factor of the dilute solution. Cf = correction factor, calculated from the effective content of the volumetric. The titer is defined as the quotient of the nominal concentration of a volumetric solution and the actual concentration. When a volumetric solution is to. Correction Factor Volumetric Solutions.
From www.youtube.com
Volumetric Analysis NUMERICALS Chemistry Class12 Important Formula NEB Correction Factor Volumetric Solutions In the latter case, the correction factor of the dilute solution. 12 rows calculation of the mass fraction w (nacl) in mg/g solution: Cf = correction factor, calculated from the effective content of the volumetric. Volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. These can be connected directly to a titrator via.. Correction Factor Volumetric Solutions.
From studylib.net
Volume Correction Factor Calculation API Correction Factor Volumetric Solutions These can be connected directly to a titrator via. Correction factor is determined on the volumetric solution to be used in the monograph. Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods. 12 rows calculation of the mass fraction w (nacl) in mg/g solution: When a volumetric solution is to be. Cf = correction factor, calculated. Correction Factor Volumetric Solutions.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1755320 Correction Factor Volumetric Solutions When a volumetric solution is to be. In the latter case, the correction factor of the dilute solution. These can be connected directly to a titrator via. 12 rows calculation of the mass fraction w (nacl) in mg/g solution: The titer is defined as the quotient of the nominal concentration of a volumetric solution and the actual concentration. Correction factor. Correction Factor Volumetric Solutions.
From www.slideshare.net
C06 concentration of solutions and volumetric analysis Correction Factor Volumetric Solutions Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods. Cf = correction factor, calculated from the effective content of the volumetric. Correction factor is determined on the volumetric solution to be used in the monograph. In the latter case, the correction factor of the dilute solution. When a volumetric solution is to be. 12 rows calculation. Correction Factor Volumetric Solutions.
From www.slideserve.com
PPT Chapter 5 Volumetric Analysis PowerPoint Presentation, free Correction Factor Volumetric Solutions Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods. These can be connected directly to a titrator via. Correction factor is determined on the volumetric solution to be used in the monograph. Volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. In the latter case, the correction factor of. Correction Factor Volumetric Solutions.
From www.narayannepal.com.np
Volumetric Analysis Class 12 Physical Chemistry Notes NEB Notes Correction Factor Volumetric Solutions 12 rows calculation of the mass fraction w (nacl) in mg/g solution: The titer is defined as the quotient of the nominal concentration of a volumetric solution and the actual concentration. Volumetric solutions are standardised by the methods described below. When a volumetric solution is to be. Volumetric analysis is a chemical analytical procedure based on measurement of volumes of. Correction Factor Volumetric Solutions.
From pt.scribd.com
Volume Correction Factor Development Petroleum Physical Quantities Correction Factor Volumetric Solutions The titer is defined as the quotient of the nominal concentration of a volumetric solution and the actual concentration. 12 rows calculation of the mass fraction w (nacl) in mg/g solution: In the latter case, the correction factor of the dilute solution. Volumetric solutions volumetric solutions are prepared according to the usual chemical analytical methods. Cf = correction factor, calculated. Correction Factor Volumetric Solutions.
From www.youtube.com
Correction Factor Intro to Statistics YouTube Correction Factor Volumetric Solutions Volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. These can be connected directly to a titrator via. 12 rows calculation of the mass fraction w (nacl) in mg/g solution: Cf = correction factor, calculated from the effective content of the volumetric. The titer is defined as the quotient of the nominal concentration. Correction Factor Volumetric Solutions.