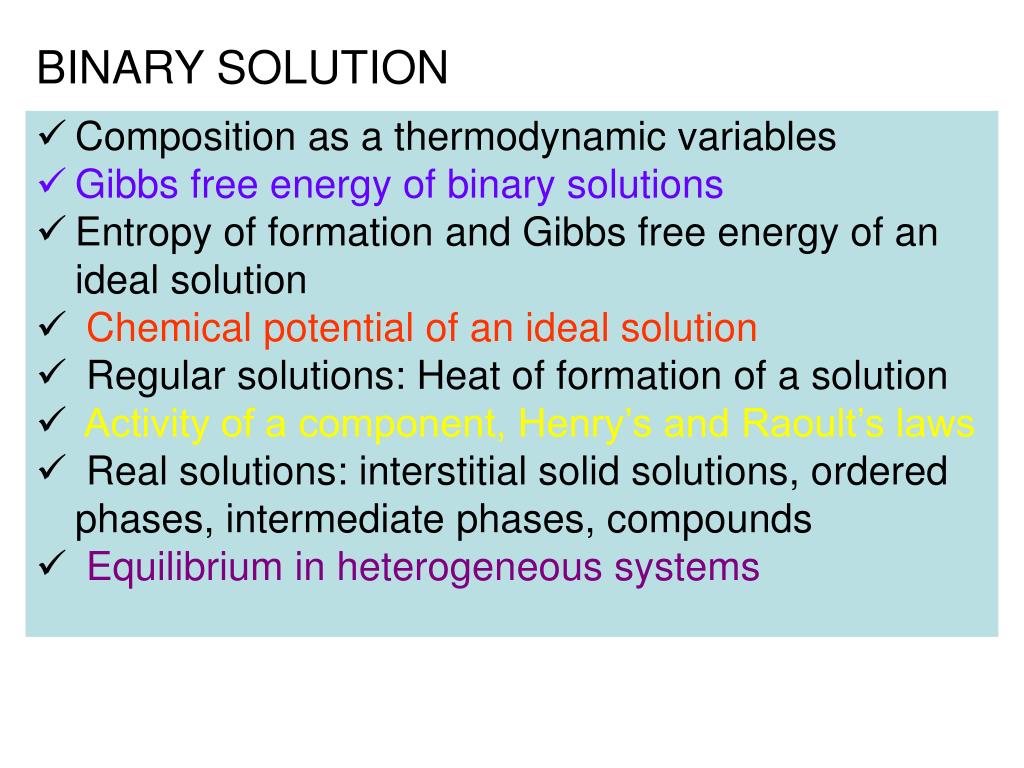
Dilute Binary Solutions . Composition of a solution can be described by expressing its concentration. A solution is called dilute when one component, called the solute, is present in. I will clarify issues frequently overlooked, touch upon solubility data reduction and correlation, report a few recent high. Consider the change in gibbs free energy when we mix two components to form a regular solution: The latter can be expressed either qualitatively or quantitatively. Our object is to look at principles applicable to all dilute solutions and, to simplify the discussion, we will consider a liquid, binary dilute solution, at. We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. A solution in thermodynamics refers to a system with more than one chemical component that is mixed homogeneously at the molecular level.
from www.slideserve.com
The latter can be expressed either qualitatively or quantitatively. Composition of a solution can be described by expressing its concentration. A solution is called dilute when one component, called the solute, is present in. A solution in thermodynamics refers to a system with more than one chemical component that is mixed homogeneously at the molecular level. We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. Our object is to look at principles applicable to all dilute solutions and, to simplify the discussion, we will consider a liquid, binary dilute solution, at. I will clarify issues frequently overlooked, touch upon solubility data reduction and correlation, report a few recent high. Consider the change in gibbs free energy when we mix two components to form a regular solution:
PPT Binary Solutions PowerPoint Presentation, free download ID4271346
Dilute Binary Solutions We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. A solution is called dilute when one component, called the solute, is present in. I will clarify issues frequently overlooked, touch upon solubility data reduction and correlation, report a few recent high. The latter can be expressed either qualitatively or quantitatively. Consider the change in gibbs free energy when we mix two components to form a regular solution: We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. A solution in thermodynamics refers to a system with more than one chemical component that is mixed homogeneously at the molecular level. Our object is to look at principles applicable to all dilute solutions and, to simplify the discussion, we will consider a liquid, binary dilute solution, at. Composition of a solution can be described by expressing its concentration.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilute Binary Solutions A solution is called dilute when one component, called the solute, is present in. I will clarify issues frequently overlooked, touch upon solubility data reduction and correlation, report a few recent high. A solution in thermodynamics refers to a system with more than one chemical component that is mixed homogeneously at the molecular level. Our object is to look at. Dilute Binary Solutions.
From www.slideserve.com
PPT Binary Solutions PowerPoint Presentation, free download ID4271346 Dilute Binary Solutions Our object is to look at principles applicable to all dilute solutions and, to simplify the discussion, we will consider a liquid, binary dilute solution, at. We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each.. Dilute Binary Solutions.
From www.youtube.com
What is Dilute Solution? Chemistry YouTube Dilute Binary Solutions The latter can be expressed either qualitatively or quantitatively. A solution is called dilute when one component, called the solute, is present in. Composition of a solution can be described by expressing its concentration. Our object is to look at principles applicable to all dilute solutions and, to simplify the discussion, we will consider a liquid, binary dilute solution, at.. Dilute Binary Solutions.
From letstalkscience.ca
Osmosis and Its Role in Human Biology and Health Let's Talk Science Dilute Binary Solutions We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. A solution in thermodynamics refers to a system with more than one chemical component that is mixed homogeneously at the molecular level. Composition of a solution. Dilute Binary Solutions.
From www.researchgate.net
(PDF) Domain of oscillatory growth in directional solidification of Dilute Binary Solutions Composition of a solution can be described by expressing its concentration. We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. A solution is called dilute when one component, called the solute, is present in. The. Dilute Binary Solutions.
From www.researchgate.net
Schematic describing the formation of a dilute nonideal solution Dilute Binary Solutions A solution is called dilute when one component, called the solute, is present in. Our object is to look at principles applicable to all dilute solutions and, to simplify the discussion, we will consider a liquid, binary dilute solution, at. We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture. Dilute Binary Solutions.
From www.youtube.com
Dilute, Concentrated and saturated solution YouTube Dilute Binary Solutions A solution in thermodynamics refers to a system with more than one chemical component that is mixed homogeneously at the molecular level. I will clarify issues frequently overlooked, touch upon solubility data reduction and correlation, report a few recent high. Consider the change in gibbs free energy when we mix two components to form a regular solution: Our object is. Dilute Binary Solutions.
From www.youtube.com
Aqueous, Dilute And Concentrated Solution, General Science Lecture Dilute Binary Solutions We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. A solution is called dilute when one component, called the solute, is present in. Composition of a solution can be described by expressing its concentration. Consider. Dilute Binary Solutions.
From www.youtube.com
Types of Solution L4 , Binary solution,Aqueous solution,Dilute Dilute Binary Solutions We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. A solution in thermodynamics refers to a system with more than one chemical component that is mixed homogeneously at the molecular level. I will clarify issues. Dilute Binary Solutions.
From learncheme.com
vaporpressureofbinarysolutions LearnChemE Dilute Binary Solutions We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. The latter can be expressed either qualitatively or quantitatively. A solution is called dilute when one component, called the solute, is present in. Our object is. Dilute Binary Solutions.
From www.slideserve.com
PPT Grade 7 Science PowerPoint Presentation, free download ID2229802 Dilute Binary Solutions The latter can be expressed either qualitatively or quantitatively. Composition of a solution can be described by expressing its concentration. Our object is to look at principles applicable to all dilute solutions and, to simplify the discussion, we will consider a liquid, binary dilute solution, at. We can use the experimental result that (for similar liquids) the mole fraction of. Dilute Binary Solutions.
From www.askiitians.com
Types Of Solutions Study Material for IIT JEE askIITians Dilute Binary Solutions We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. I will clarify issues frequently overlooked, touch upon solubility data reduction and correlation, report a few recent high. Our object is to look at principles applicable. Dilute Binary Solutions.
From www.youtube.com
concentrated and dilute solution binary solution solution Dilute Binary Solutions A solution is called dilute when one component, called the solute, is present in. Consider the change in gibbs free energy when we mix two components to form a regular solution: We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the. Dilute Binary Solutions.
From www.slideserve.com
PPT Binary Solutions PowerPoint Presentation, free download ID3591081 Dilute Binary Solutions A solution in thermodynamics refers to a system with more than one chemical component that is mixed homogeneously at the molecular level. Consider the change in gibbs free energy when we mix two components to form a regular solution: Our object is to look at principles applicable to all dilute solutions and, to simplify the discussion, we will consider a. Dilute Binary Solutions.
From www.slideserve.com
PPT Grade 7 Science PowerPoint Presentation, free download ID2229802 Dilute Binary Solutions Composition of a solution can be described by expressing its concentration. Consider the change in gibbs free energy when we mix two components to form a regular solution: We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures. Dilute Binary Solutions.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Binary Solutions A solution is called dilute when one component, called the solute, is present in. Composition of a solution can be described by expressing its concentration. I will clarify issues frequently overlooked, touch upon solubility data reduction and correlation, report a few recent high. We can use the experimental result that (for similar liquids) the mole fraction of the substance a. Dilute Binary Solutions.
From www.youtube.com
Lecture 9 (5 of 5) Dilute Solutions YouTube Dilute Binary Solutions A solution is called dilute when one component, called the solute, is present in. Our object is to look at principles applicable to all dilute solutions and, to simplify the discussion, we will consider a liquid, binary dilute solution, at. We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture. Dilute Binary Solutions.
From www.askdifference.com
Dilute Solution vs. Concentrated Solution — What’s the Difference? Dilute Binary Solutions I will clarify issues frequently overlooked, touch upon solubility data reduction and correlation, report a few recent high. We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. Our object is to look at principles applicable. Dilute Binary Solutions.
From www.slideserve.com
PPT Binary Solutions PowerPoint Presentation, free download ID4271346 Dilute Binary Solutions I will clarify issues frequently overlooked, touch upon solubility data reduction and correlation, report a few recent high. A solution in thermodynamics refers to a system with more than one chemical component that is mixed homogeneously at the molecular level. The latter can be expressed either qualitatively or quantitatively. Consider the change in gibbs free energy when we mix two. Dilute Binary Solutions.
From www.slideserve.com
PPT Binary Solutions PowerPoint Presentation, free download ID4271346 Dilute Binary Solutions Composition of a solution can be described by expressing its concentration. Consider the change in gibbs free energy when we mix two components to form a regular solution: I will clarify issues frequently overlooked, touch upon solubility data reduction and correlation, report a few recent high. We can use the experimental result that (for similar liquids) the mole fraction of. Dilute Binary Solutions.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Binary Solutions Composition of a solution can be described by expressing its concentration. A solution is called dilute when one component, called the solute, is present in. I will clarify issues frequently overlooked, touch upon solubility data reduction and correlation, report a few recent high. We can use the experimental result that (for similar liquids) the mole fraction of the substance a. Dilute Binary Solutions.
From www.slideserve.com
PPT Binary Solutions PowerPoint Presentation, free download ID4271346 Dilute Binary Solutions I will clarify issues frequently overlooked, touch upon solubility data reduction and correlation, report a few recent high. We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. The latter can be expressed either qualitatively or. Dilute Binary Solutions.
From www.pinterest.de
Dilution when solvent is added to dilute a solution, the number of Dilute Binary Solutions A solution in thermodynamics refers to a system with more than one chemical component that is mixed homogeneously at the molecular level. Our object is to look at principles applicable to all dilute solutions and, to simplify the discussion, we will consider a liquid, binary dilute solution, at. We can use the experimental result that (for similar liquids) the mole. Dilute Binary Solutions.
From www.slideserve.com
PPT Chapter 15 Solutions PowerPoint Presentation, free download ID Dilute Binary Solutions A solution in thermodynamics refers to a system with more than one chemical component that is mixed homogeneously at the molecular level. The latter can be expressed either qualitatively or quantitatively. Consider the change in gibbs free energy when we mix two components to form a regular solution: Our object is to look at principles applicable to all dilute solutions. Dilute Binary Solutions.
From www.pdfprof.com
how to dilute solutions Dilute Binary Solutions Our object is to look at principles applicable to all dilute solutions and, to simplify the discussion, we will consider a liquid, binary dilute solution, at. The latter can be expressed either qualitatively or quantitatively. A solution is called dilute when one component, called the solute, is present in. We can use the experimental result that (for similar liquids) the. Dilute Binary Solutions.
From www.slideserve.com
PPT Solutions Part II PowerPoint Presentation, free download ID4272422 Dilute Binary Solutions A solution is called dilute when one component, called the solute, is present in. I will clarify issues frequently overlooked, touch upon solubility data reduction and correlation, report a few recent high. A solution in thermodynamics refers to a system with more than one chemical component that is mixed homogeneously at the molecular level. Our object is to look at. Dilute Binary Solutions.
From www.slideserve.com
PPT Binary Solutions PowerPoint Presentation, free download ID4271346 Dilute Binary Solutions I will clarify issues frequently overlooked, touch upon solubility data reduction and correlation, report a few recent high. The latter can be expressed either qualitatively or quantitatively. A solution is called dilute when one component, called the solute, is present in. Consider the change in gibbs free energy when we mix two components to form a regular solution: Composition of. Dilute Binary Solutions.
From www.researchgate.net
The most stable geometry for dilute binary surface alloys with a DFT Dilute Binary Solutions A solution in thermodynamics refers to a system with more than one chemical component that is mixed homogeneously at the molecular level. Composition of a solution can be described by expressing its concentration. Consider the change in gibbs free energy when we mix two components to form a regular solution: I will clarify issues frequently overlooked, touch upon solubility data. Dilute Binary Solutions.
From www.researchgate.net
Measured integral enthalpy of formation of liquid dilute binary AlZr Dilute Binary Solutions The latter can be expressed either qualitatively or quantitatively. I will clarify issues frequently overlooked, touch upon solubility data reduction and correlation, report a few recent high. Our object is to look at principles applicable to all dilute solutions and, to simplify the discussion, we will consider a liquid, binary dilute solution, at. Composition of a solution can be described. Dilute Binary Solutions.
From aiche.onlinelibrary.wiley.com
General behavior of dilute binary solutions Cochran 1987 AIChE Dilute Binary Solutions We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. A solution in thermodynamics refers to a system with more than one chemical component that is mixed homogeneously at the molecular level. A solution is called. Dilute Binary Solutions.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID398040 Dilute Binary Solutions A solution in thermodynamics refers to a system with more than one chemical component that is mixed homogeneously at the molecular level. The latter can be expressed either qualitatively or quantitatively. Our object is to look at principles applicable to all dilute solutions and, to simplify the discussion, we will consider a liquid, binary dilute solution, at. Consider the change. Dilute Binary Solutions.
From www.youtube.com
Integral Enthalpies of Solution and Dilution & Enthalpy of Solution at Dilute Binary Solutions Composition of a solution can be described by expressing its concentration. A solution is called dilute when one component, called the solute, is present in. A solution in thermodynamics refers to a system with more than one chemical component that is mixed homogeneously at the molecular level. Consider the change in gibbs free energy when we mix two components to. Dilute Binary Solutions.
From www.chegg.com
Solved A dilute binary alloy of overall composition Co is Dilute Binary Solutions I will clarify issues frequently overlooked, touch upon solubility data reduction and correlation, report a few recent high. A solution in thermodynamics refers to a system with more than one chemical component that is mixed homogeneously at the molecular level. We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture. Dilute Binary Solutions.
From ar.inspiredpencil.com
Dilute Solution Diagram Dilute Binary Solutions Consider the change in gibbs free energy when we mix two components to form a regular solution: The latter can be expressed either qualitatively or quantitatively. I will clarify issues frequently overlooked, touch upon solubility data reduction and correlation, report a few recent high. Composition of a solution can be described by expressing its concentration. Our object is to look. Dilute Binary Solutions.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilute Binary Solutions We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. I will clarify issues frequently overlooked, touch upon solubility data reduction and correlation, report a few recent high. Composition of a solution can be described by. Dilute Binary Solutions.