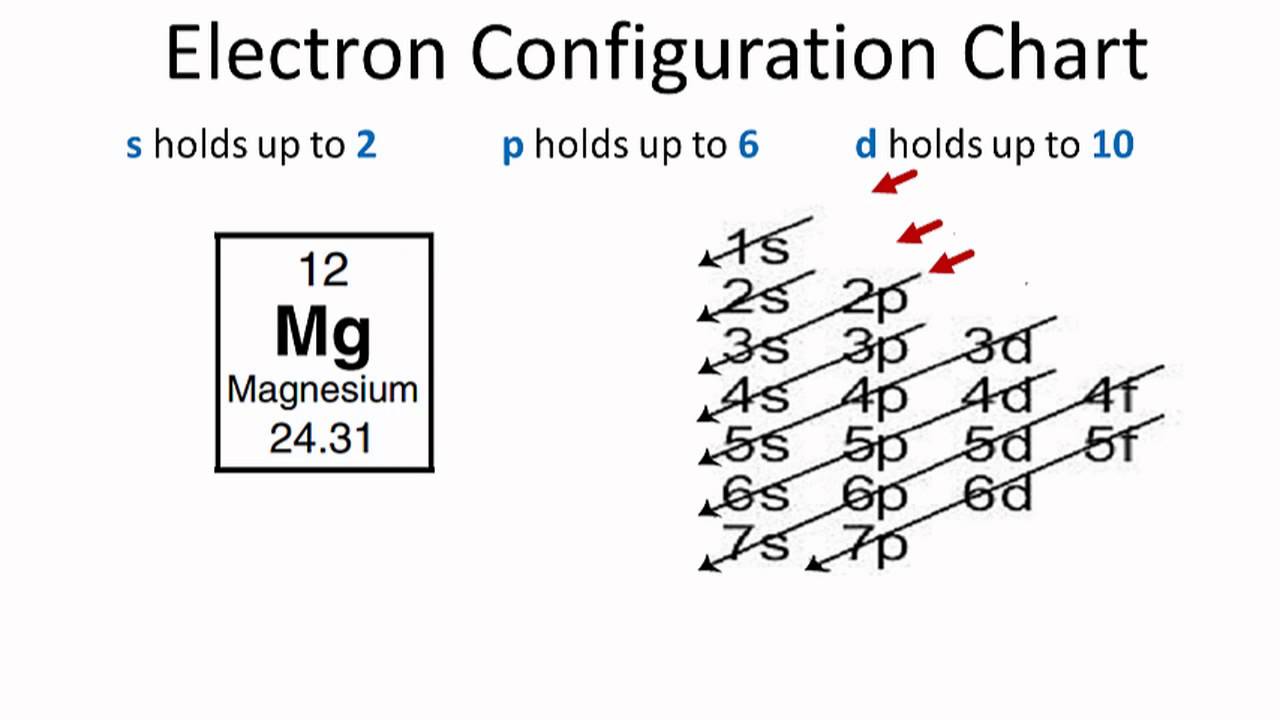
Magnesium Sulfate Valence Electrons . Determine the total number of valence electrons in the molecule or ion. Describe the stability of an atom as a result of following the octet rule. Each h atom (group 1) has 1 valence electron, and the o. Valence electrons determine the reactivity of an atom. A metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a positive. Explain the relevance of valence electrons in chemical processes. The electrons on the outermost energy level of the atom are called valence electrons. The valence electrons are involved in bonding one atom to another. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Identify the number of valence electrons in an element. But for most of the transition.
from periodictable.me
But for most of the transition. Describe the stability of an atom as a result of following the octet rule. A metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a positive. The electrons on the outermost energy level of the atom are called valence electrons. Determine the total number of valence electrons in the molecule or ion. Identify the number of valence electrons in an element. Each h atom (group 1) has 1 valence electron, and the o. Valence electrons determine the reactivity of an atom. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The valence electrons are involved in bonding one atom to another.
Magnesium Electron Configuration (Mg) with Orbital Diagram
Magnesium Sulfate Valence Electrons The electrons on the outermost energy level of the atom are called valence electrons. The valence electrons are involved in bonding one atom to another. The electrons on the outermost energy level of the atom are called valence electrons. A metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a positive. Determine the total number of valence electrons in the molecule or ion. Describe the stability of an atom as a result of following the octet rule. Explain the relevance of valence electrons in chemical processes. Identify the number of valence electrons in an element. But for most of the transition. Valence electrons determine the reactivity of an atom. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Each h atom (group 1) has 1 valence electron, and the o.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Magnesium Sulfate Valence Electrons Identify the number of valence electrons in an element. Each h atom (group 1) has 1 valence electron, and the o. Explain the relevance of valence electrons in chemical processes. A metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a positive. But for most of the transition. The. Magnesium Sulfate Valence Electrons.
From stock.adobe.com
Molecular formula and chemical structure of magnesium sulfate vector de Magnesium Sulfate Valence Electrons Identify the number of valence electrons in an element. Explain the relevance of valence electrons in chemical processes. A metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a positive. The valence electrons are involved in bonding one atom to another. The electrons on the outermost energy level of. Magnesium Sulfate Valence Electrons.
From www.shutterstock.com
Magnesium Sulfate Molecule Structure Stock Vector (Royalty Free Magnesium Sulfate Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Identify the number of valence electrons in an element. But for most of the transition. A metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form. Magnesium Sulfate Valence Electrons.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Magnesium Sulfate Valence Electrons Each h atom (group 1) has 1 valence electron, and the o. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The valence electrons are involved in bonding one atom to another. A metal from group 2 (e.g., magnesium) is somewhat less reactive, because each. Magnesium Sulfate Valence Electrons.
From mavink.com
Magnesium Atomic Structure Magnesium Sulfate Valence Electrons Each h atom (group 1) has 1 valence electron, and the o. Describe the stability of an atom as a result of following the octet rule. But for most of the transition. Explain the relevance of valence electrons in chemical processes. The electrons on the outermost energy level of the atom are called valence electrons. Identify the number of valence. Magnesium Sulfate Valence Electrons.
From www.dreamstime.com
3D Image of Magnesium Sulfate Skeletal Formula Stock Illustration Magnesium Sulfate Valence Electrons But for most of the transition. The valence electrons are involved in bonding one atom to another. Valence electrons determine the reactivity of an atom. The electrons on the outermost energy level of the atom are called valence electrons. Each h atom (group 1) has 1 valence electron, and the o. Determine the total number of valence electrons in the. Magnesium Sulfate Valence Electrons.
From www.alamy.com
3D image of Magnesium sulfate skeletal formula molecular chemical Magnesium Sulfate Valence Electrons But for most of the transition. The electrons on the outermost energy level of the atom are called valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Each h atom (group 1) has 1 valence electron, and the o. Determine the total number. Magnesium Sulfate Valence Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Magnesium (Mg)? Magnesium Sulfate Valence Electrons Each h atom (group 1) has 1 valence electron, and the o. Valence electrons determine the reactivity of an atom. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Determine the total number of valence electrons in the molecule or ion. But for most of. Magnesium Sulfate Valence Electrons.
From www.youtube.com
How to Draw the Lewis Dot Structure for MgSO4 Magnesium sulfate YouTube Magnesium Sulfate Valence Electrons But for most of the transition. Each h atom (group 1) has 1 valence electron, and the o. Identify the number of valence electrons in an element. The valence electrons are involved in bonding one atom to another. Determine the total number of valence electrons in the molecule or ion. Explain the relevance of valence electrons in chemical processes. Valence. Magnesium Sulfate Valence Electrons.
From www.dreamstime.com
Magnesium Sulfate, Salt, Molecular Structures, 3d Model, Structural Magnesium Sulfate Valence Electrons Explain the relevance of valence electrons in chemical processes. Each h atom (group 1) has 1 valence electron, and the o. The valence electrons are involved in bonding one atom to another. Determine the total number of valence electrons in the molecule or ion. The electrons on the outermost energy level of the atom are called valence electrons. But for. Magnesium Sulfate Valence Electrons.
From valenceelectrons.com
Electron Configuration for Magnesium(Mg, Mg2+ ion) Magnesium Sulfate Valence Electrons A metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a positive. The valence electrons are involved in bonding one atom to another. Identify the number of valence electrons in an element. Explain the relevance of valence electrons in chemical processes. But for most of the transition. Determine the. Magnesium Sulfate Valence Electrons.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo Magnesium Sulfate Valence Electrons A metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a positive. Explain the relevance of valence electrons in chemical processes. Describe the stability of an atom as a result of following the octet rule. Each h atom (group 1) has 1 valence electron, and the o. Valence electrons. Magnesium Sulfate Valence Electrons.
From nursehub.com
Electron Shells NurseHub Magnesium Sulfate Valence Electrons Identify the number of valence electrons in an element. Determine the total number of valence electrons in the molecule or ion. Explain the relevance of valence electrons in chemical processes. Valence electrons determine the reactivity of an atom. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. Magnesium Sulfate Valence Electrons.
From www.dreamstime.com
Atom Of Magnesium With Detailed Core And Its 12 Electrons Stock Magnesium Sulfate Valence Electrons Describe the stability of an atom as a result of following the octet rule. Valence electrons determine the reactivity of an atom. Each h atom (group 1) has 1 valence electron, and the o. The electrons on the outermost energy level of the atom are called valence electrons. Identify the number of valence electrons in an element. Determine the total. Magnesium Sulfate Valence Electrons.
From slidetodoc.com
Ionic Bonding Elements are the simplest substances There Magnesium Sulfate Valence Electrons Identify the number of valence electrons in an element. But for most of the transition. Explain the relevance of valence electrons in chemical processes. A metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a positive. Describe the stability of an atom as a result of following the octet. Magnesium Sulfate Valence Electrons.
From www.bigstockphoto.com
3d Render Atom Structure Magnesium Image & Photo Bigstock Magnesium Sulfate Valence Electrons A metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a positive. Describe the stability of an atom as a result of following the octet rule. The valence electrons are involved in bonding one atom to another. Explain the relevance of valence electrons in chemical processes. But for most. Magnesium Sulfate Valence Electrons.
From sites.google.com
Atomic Structure Protons, Neutrons and Electrons Mrs. Sanborn's Site Magnesium Sulfate Valence Electrons The electrons on the outermost energy level of the atom are called valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The valence electrons are involved in bonding one atom to another. Determine the total number of valence electrons in the molecule or. Magnesium Sulfate Valence Electrons.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Magnesium Sulfate Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Describe the stability of an atom as a result of following the octet rule. The valence electrons are involved in bonding one atom to another. But for most of the transition. A metal from group 2. Magnesium Sulfate Valence Electrons.
From www.slideserve.com
PPT How many valence electrons does magnesium have? PowerPoint Magnesium Sulfate Valence Electrons The electrons on the outermost energy level of the atom are called valence electrons. The valence electrons are involved in bonding one atom to another. But for most of the transition. Valence electrons determine the reactivity of an atom. Identify the number of valence electrons in an element. 93 rows you may assume the valences of the chemical elements—the number. Magnesium Sulfate Valence Electrons.
From worldupdatereviews.com
Properties of Magnesium Sulfate World Update Review Magnesium Sulfate Valence Electrons Explain the relevance of valence electrons in chemical processes. A metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a positive. The valence electrons are involved in bonding one atom to another. Describe the stability of an atom as a result of following the octet rule. Determine the total. Magnesium Sulfate Valence Electrons.
From periodictable.me
Magnesium Valence Electron Magnesium Valency (Mg) with Dot Diagram Magnesium Sulfate Valence Electrons The electrons on the outermost energy level of the atom are called valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Describe the stability of an atom as a result of following the octet rule. Explain the relevance of valence electrons in chemical. Magnesium Sulfate Valence Electrons.
From www.slideserve.com
PPT Valence Electrons and Oxidation Numbers PowerPoint Presentation Magnesium Sulfate Valence Electrons But for most of the transition. The electrons on the outermost energy level of the atom are called valence electrons. Identify the number of valence electrons in an element. Each h atom (group 1) has 1 valence electron, and the o. The valence electrons are involved in bonding one atom to another. A metal from group 2 (e.g., magnesium) is. Magnesium Sulfate Valence Electrons.
From www.youtube.com
Valence Electrons for Magnesium (Mg) YouTube Magnesium Sulfate Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. But for most of the transition. Explain the relevance of valence electrons in chemical processes. The valence electrons are involved in bonding one atom to another. The electrons on the outermost energy level of the atom. Magnesium Sulfate Valence Electrons.
From www.pinterest.co.uk
Magnesium, atomic structure Stock Image C018/3693 Science Photo Magnesium Sulfate Valence Electrons Valence electrons determine the reactivity of an atom. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. A metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a positive. But for most of the. Magnesium Sulfate Valence Electrons.
From www.smartscience.co.th
Magnesium sulfate, anhydrous smartscience Magnesium Sulfate Valence Electrons The valence electrons are involved in bonding one atom to another. But for most of the transition. Determine the total number of valence electrons in the molecule or ion. Valence electrons determine the reactivity of an atom. Explain the relevance of valence electrons in chemical processes. Identify the number of valence electrons in an element. A metal from group 2. Magnesium Sulfate Valence Electrons.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Sulfate Valence Electrons Valence electrons determine the reactivity of an atom. The valence electrons are involved in bonding one atom to another. Determine the total number of valence electrons in the molecule or ion. But for most of the transition. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those. Magnesium Sulfate Valence Electrons.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Sulfate Valence Electrons Determine the total number of valence electrons in the molecule or ion. Each h atom (group 1) has 1 valence electron, and the o. Describe the stability of an atom as a result of following the octet rule. The valence electrons are involved in bonding one atom to another. Identify the number of valence electrons in an element. Explain the. Magnesium Sulfate Valence Electrons.
From valenceelectrons.com
Magnesium(Mg) electron configuration and orbital diagram Magnesium Sulfate Valence Electrons Determine the total number of valence electrons in the molecule or ion. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Identify the number of valence electrons in an element. A metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must. Magnesium Sulfate Valence Electrons.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Sulfate Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The valence electrons are involved in bonding one atom to another. The electrons on the outermost energy level of the atom are called valence electrons. But for most of the transition. Valence electrons determine the reactivity. Magnesium Sulfate Valence Electrons.
From www.vectorstock.com
Symbol and electron diagram for magnesium Vector Image Magnesium Sulfate Valence Electrons The valence electrons are involved in bonding one atom to another. Each h atom (group 1) has 1 valence electron, and the o. But for most of the transition. A metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a positive. Valence electrons determine the reactivity of an atom.. Magnesium Sulfate Valence Electrons.
From www.alamy.com
Magnesium sulfate molecule. It is is an salt and Magnesium Sulfate Valence Electrons Explain the relevance of valence electrons in chemical processes. A metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a positive. Describe the stability of an atom as a result of following the octet rule. Valence electrons determine the reactivity of an atom. Each h atom (group 1) has. Magnesium Sulfate Valence Electrons.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Sulfate Valence Electrons Determine the total number of valence electrons in the molecule or ion. The electrons on the outermost energy level of the atom are called valence electrons. Each h atom (group 1) has 1 valence electron, and the o. The valence electrons are involved in bonding one atom to another. 93 rows you may assume the valences of the chemical elements—the. Magnesium Sulfate Valence Electrons.
From www.slideserve.com
PPT How many valence electrons does magnesium have? PowerPoint Magnesium Sulfate Valence Electrons Describe the stability of an atom as a result of following the octet rule. But for most of the transition. Determine the total number of valence electrons in the molecule or ion. The electrons on the outermost energy level of the atom are called valence electrons. Valence electrons determine the reactivity of an atom. Explain the relevance of valence electrons. Magnesium Sulfate Valence Electrons.
From periodictable.me
Magnesium Valence Electron Magnesium Valency (Mg) with Dot Diagram Magnesium Sulfate Valence Electrons Explain the relevance of valence electrons in chemical processes. A metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a positive. Describe the stability of an atom as a result of following the octet rule. Each h atom (group 1) has 1 valence electron, and the o. 93 rows. Magnesium Sulfate Valence Electrons.
From www.youtube.com
Mg Orbital Diagram How to Write the Atomic Orbital Diagram for Magnesium Sulfate Valence Electrons The valence electrons are involved in bonding one atom to another. A metal from group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a positive. Determine the total number of valence electrons in the molecule or ion. Each h atom (group 1) has 1 valence electron, and the o. The electrons. Magnesium Sulfate Valence Electrons.