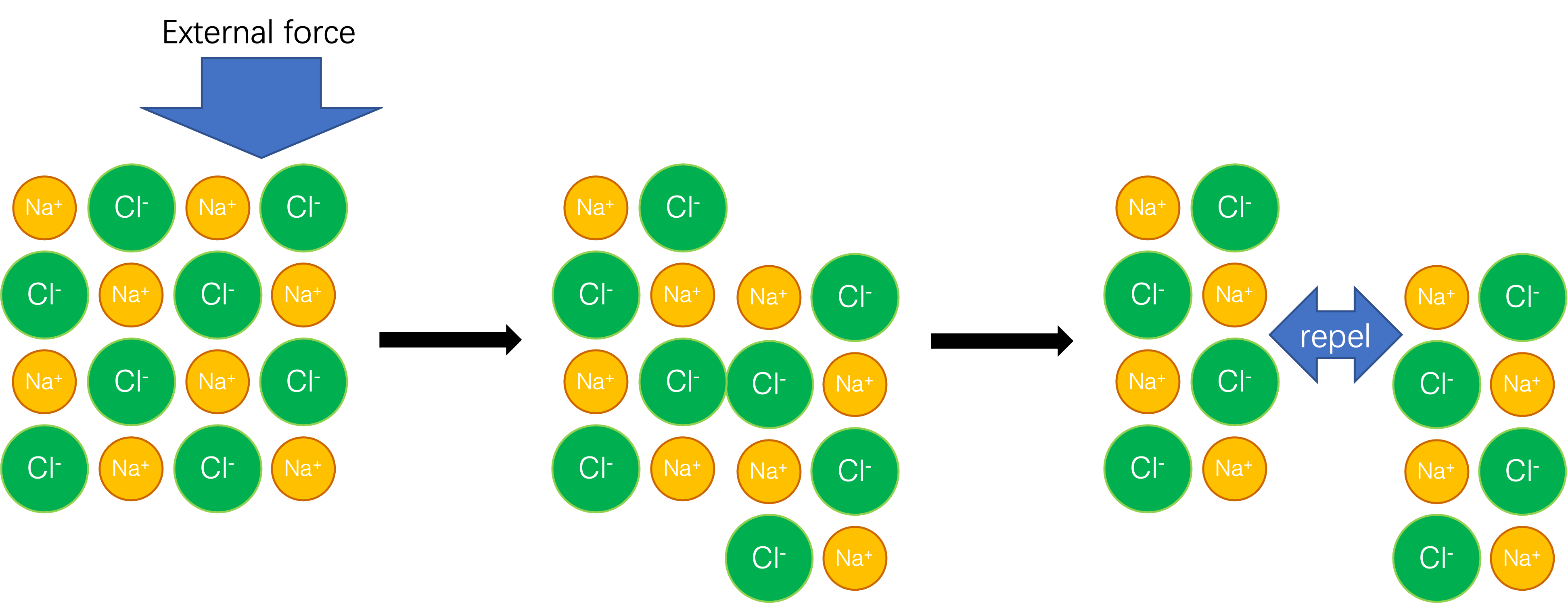
What Is Brittle In Chemistry . For engineers, the understanding of the difference between brittle. Brittleness refers to a material’s tendency to fracture or shatter when subjected to stress or impact. We have defined brittleness of polymeric materials quantitatively with applications to multiple areas. Why are melting points high for ionic compounds? What happens when an electric current is passed through a solution of an ionic compound? In materials science, brittleness is understood as the lack of ductility. A brittle substance has no elasticity and shows little deformation before. Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. Since in ceramics the rows cannot slide, the ceramic. In metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. Why are ionic compounds brittle? Brittleness describes the property of a material that fractures when subjected to stress but has a little tendency to deform before rupture.
from ibstudy.net
What happens when an electric current is passed through a solution of an ionic compound? We have defined brittleness of polymeric materials quantitatively with applications to multiple areas. A brittle substance has no elasticity and shows little deformation before. Brittleness refers to a material’s tendency to fracture or shatter when subjected to stress or impact. Brittleness describes the property of a material that fractures when subjected to stress but has a little tendency to deform before rupture. Since in ceramics the rows cannot slide, the ceramic. In materials science, brittleness is understood as the lack of ductility. Why are melting points high for ionic compounds? In metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. For engineers, the understanding of the difference between brittle.
4.1 Ionic bonding and structure Wang's website
What Is Brittle In Chemistry For engineers, the understanding of the difference between brittle. Brittleness describes the property of a material that fractures when subjected to stress but has a little tendency to deform before rupture. For engineers, the understanding of the difference between brittle. Brittleness refers to a material’s tendency to fracture or shatter when subjected to stress or impact. In metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. We have defined brittleness of polymeric materials quantitatively with applications to multiple areas. Since in ceramics the rows cannot slide, the ceramic. A brittle substance has no elasticity and shows little deformation before. In materials science, brittleness is understood as the lack of ductility. Why are ionic compounds brittle? What happens when an electric current is passed through a solution of an ionic compound? Why are melting points high for ionic compounds?
From www.youtube.com
Ionic Bonding Properties of Ionic Compounds YouTube What Is Brittle In Chemistry Why are ionic compounds brittle? In materials science, brittleness is understood as the lack of ductility. Brittleness refers to a material’s tendency to fracture or shatter when subjected to stress or impact. Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. What happens when an electric. What Is Brittle In Chemistry.
From www.vedantu.com
Explain with examples, ductile materials, brittle materials and What Is Brittle In Chemistry A brittle substance has no elasticity and shows little deformation before. Brittleness describes the property of a material that fractures when subjected to stress but has a little tendency to deform before rupture. Why are ionic compounds brittle? In metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. In. What Is Brittle In Chemistry.
From exocrbooc.blob.core.windows.net
Brittle Chemistry at Manuel Whitt blog What Is Brittle In Chemistry Brittleness refers to a material’s tendency to fracture or shatter when subjected to stress or impact. Why are ionic compounds brittle? A brittle substance has no elasticity and shows little deformation before. What happens when an electric current is passed through a solution of an ionic compound? In materials science, brittleness is understood as the lack of ductility. Brittleness is. What Is Brittle In Chemistry.
From www.youtube.com
Ductile and Brittle Materials by stress strain curve YouTube What Is Brittle In Chemistry Brittleness describes the property of a material that fractures when subjected to stress but has a little tendency to deform before rupture. A brittle substance has no elasticity and shows little deformation before. In materials science, brittleness is understood as the lack of ductility. What happens when an electric current is passed through a solution of an ionic compound? Why. What Is Brittle In Chemistry.
From www.gauthmath.com
Solved What type of element is brittle and acts as an insulator? Metal What Is Brittle In Chemistry Since in ceramics the rows cannot slide, the ceramic. Why are melting points high for ionic compounds? Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. Brittleness describes the property of a material that fractures when subjected to stress but has a little tendency to deform. What Is Brittle In Chemistry.
From libatoms.github.io
Theoretical background — quippy 7c3fe3b documentation What Is Brittle In Chemistry Since in ceramics the rows cannot slide, the ceramic. In materials science, brittleness is understood as the lack of ductility. Brittleness describes the property of a material that fractures when subjected to stress but has a little tendency to deform before rupture. In metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically. What Is Brittle In Chemistry.
From www.instructables.com
Peanut Brittle Chemistry Lab (with Pictures) Instructables What Is Brittle In Chemistry Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. In metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. Brittleness refers to a material’s tendency to fracture or shatter when subjected to stress or impact.. What Is Brittle In Chemistry.
From blog.thepipingmart.com
Explain In Detail The Concept Of Brittleness ThePipingMart Blog What Is Brittle In Chemistry Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. We have defined brittleness of polymeric materials quantitatively with applications to multiple areas. Brittleness refers to a material’s tendency to fracture or shatter when subjected to stress or impact. What happens when an electric current is passed. What Is Brittle In Chemistry.
From howforkids.com
15 Examples of Brittle Materials HowForKids What Is Brittle In Chemistry Why are ionic compounds brittle? A brittle substance has no elasticity and shows little deformation before. Brittleness refers to a material’s tendency to fracture or shatter when subjected to stress or impact. In materials science, brittleness is understood as the lack of ductility. What happens when an electric current is passed through a solution of an ionic compound? Why are. What Is Brittle In Chemistry.
From www.slideserve.com
PPT MYP Chemistry Ionic Bonding and Ionic Compounds PowerPoint What Is Brittle In Chemistry For engineers, the understanding of the difference between brittle. Since in ceramics the rows cannot slide, the ceramic. Why are ionic compounds brittle? Brittleness refers to a material’s tendency to fracture or shatter when subjected to stress or impact. We have defined brittleness of polymeric materials quantitatively with applications to multiple areas. A brittle substance has no elasticity and shows. What Is Brittle In Chemistry.
From whatispiping.com
Brittle Fracture and Ductile Fracture Definition, Mechanism What Is Brittle In Chemistry We have defined brittleness of polymeric materials quantitatively with applications to multiple areas. Brittleness describes the property of a material that fractures when subjected to stress but has a little tendency to deform before rupture. Since in ceramics the rows cannot slide, the ceramic. Brittleness refers to a material’s tendency to fracture or shatter when subjected to stress or impact.. What Is Brittle In Chemistry.
From www.youtube.com
Why is concrete so brittle? Chemistry Materials How they Work What Is Brittle In Chemistry In materials science, brittleness is understood as the lack of ductility. Why are melting points high for ionic compounds? For engineers, the understanding of the difference between brittle. A brittle substance has no elasticity and shows little deformation before. Why are ionic compounds brittle? We have defined brittleness of polymeric materials quantitatively with applications to multiple areas. Brittleness refers to. What Is Brittle In Chemistry.
From ibstudy.net
4.1 Ionic bonding and structure Wang's website What Is Brittle In Chemistry A brittle substance has no elasticity and shows little deformation before. Why are ionic compounds brittle? In materials science, brittleness is understood as the lack of ductility. Why are melting points high for ionic compounds? Since in ceramics the rows cannot slide, the ceramic. What happens when an electric current is passed through a solution of an ionic compound? For. What Is Brittle In Chemistry.
From www.bbc.co.uk
Chemistry KS3 & KS4/GCSE Why is concrete so brittle? BBC Teach What Is Brittle In Chemistry Brittleness describes the property of a material that fractures when subjected to stress but has a little tendency to deform before rupture. Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. Since in ceramics the rows cannot slide, the ceramic. Brittleness refers to a material’s tendency. What Is Brittle In Chemistry.
From www.chemistry-teaching-resources.com
chemistry picture What Is Brittle In Chemistry Since in ceramics the rows cannot slide, the ceramic. Why are melting points high for ionic compounds? Brittleness refers to a material’s tendency to fracture or shatter when subjected to stress or impact. In metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. Brittleness describes the property of a. What Is Brittle In Chemistry.
From pediaa.com
Difference Between Ductile and Brittle Definition, Examples, Effect What Is Brittle In Chemistry In metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. We have defined brittleness of polymeric materials quantitatively with applications to multiple areas. A brittle substance has no elasticity and shows little deformation before. Why are melting points high for ionic compounds? Brittleness is the opposite of ductility, in. What Is Brittle In Chemistry.
From www.fictiv.com
What are Brittle Materials? Fictiv What Is Brittle In Chemistry Why are melting points high for ionic compounds? Since in ceramics the rows cannot slide, the ceramic. Brittleness describes the property of a material that fractures when subjected to stress but has a little tendency to deform before rupture. Brittleness refers to a material’s tendency to fracture or shatter when subjected to stress or impact. For engineers, the understanding of. What Is Brittle In Chemistry.
From www.breakingatom.com
Brittle Definition What Is Brittle In Chemistry A brittle substance has no elasticity and shows little deformation before. For engineers, the understanding of the difference between brittle. What happens when an electric current is passed through a solution of an ionic compound? We have defined brittleness of polymeric materials quantitatively with applications to multiple areas. In metals, the sliding of rows of atoms results in slip, which. What Is Brittle In Chemistry.
From exoblumsd.blob.core.windows.net
Brittle In Chemistry at Charles Myrick blog What Is Brittle In Chemistry In metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. In materials science, brittleness is understood as the lack of ductility. For engineers, the understanding of the difference between brittle. A brittle substance has no elasticity and shows little deformation before. Brittleness is the opposite of ductility, in which. What Is Brittle In Chemistry.
From www.researchgate.net
Brittle vs. ductile behavior in materials. Download Scientific Diagram What Is Brittle In Chemistry Why are melting points high for ionic compounds? Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. For engineers, the understanding of the difference between brittle. In metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of. What Is Brittle In Chemistry.
From blog.thepipingmart.com
Is Zinc a Brittle Metal? What Is Brittle In Chemistry Brittleness describes the property of a material that fractures when subjected to stress but has a little tendency to deform before rupture. In materials science, brittleness is understood as the lack of ductility. Why are ionic compounds brittle? A brittle substance has no elasticity and shows little deformation before. Brittleness refers to a material’s tendency to fracture or shatter when. What Is Brittle In Chemistry.
From www.slideserve.com
PPT Chapter 15 Properties of Matter PowerPoint Presentation, free What Is Brittle In Chemistry Why are ionic compounds brittle? Since in ceramics the rows cannot slide, the ceramic. Why are melting points high for ionic compounds? For engineers, the understanding of the difference between brittle. In metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. We have defined brittleness of polymeric materials quantitatively. What Is Brittle In Chemistry.
From pediaa.com
Difference Between Ductile and Brittle Definition, Examples, Effect What Is Brittle In Chemistry Why are ionic compounds brittle? Why are melting points high for ionic compounds? We have defined brittleness of polymeric materials quantitatively with applications to multiple areas. For engineers, the understanding of the difference between brittle. In metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. Since in ceramics the. What Is Brittle In Chemistry.
From www.slideserve.com
PPT PROPERTIES OF MATTER PowerPoint Presentation, free download ID What Is Brittle In Chemistry Brittleness describes the property of a material that fractures when subjected to stress but has a little tendency to deform before rupture. In materials science, brittleness is understood as the lack of ductility. For engineers, the understanding of the difference between brittle. In metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically. What Is Brittle In Chemistry.
From www.gauthmath.com
Solved A solid is brittle, has a low melting point, and has the What Is Brittle In Chemistry Why are melting points high for ionic compounds? Brittleness refers to a material’s tendency to fracture or shatter when subjected to stress or impact. Brittleness describes the property of a material that fractures when subjected to stress but has a little tendency to deform before rupture. In materials science, brittleness is understood as the lack of ductility. A brittle substance. What Is Brittle In Chemistry.
From exoblumsd.blob.core.windows.net
Brittle In Chemistry at Charles Myrick blog What Is Brittle In Chemistry Brittleness refers to a material’s tendency to fracture or shatter when subjected to stress or impact. Why are ionic compounds brittle? Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. Since in ceramics the rows cannot slide, the ceramic. Why are melting points high for ionic. What Is Brittle In Chemistry.
From www.slideserve.com
PPT Classification of Matter PowerPoint Presentation, free download What Is Brittle In Chemistry For engineers, the understanding of the difference between brittle. Brittleness refers to a material’s tendency to fracture or shatter when subjected to stress or impact. We have defined brittleness of polymeric materials quantitatively with applications to multiple areas. In metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. In. What Is Brittle In Chemistry.
From www.researchgate.net
4) Photomicrographs show the mineral composition and brittleductile What Is Brittle In Chemistry Brittleness describes the property of a material that fractures when subjected to stress but has a little tendency to deform before rupture. Why are ionic compounds brittle? In materials science, brittleness is understood as the lack of ductility. Why are melting points high for ionic compounds? For engineers, the understanding of the difference between brittle. Since in ceramics the rows. What Is Brittle In Chemistry.
From insidescience.org
BRIEF A Brittle Crystal Flexible in the Dark Inside Science What Is Brittle In Chemistry Why are melting points high for ionic compounds? Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. Brittleness describes the property of a material that fractures when subjected to stress but has a little tendency to deform before rupture. For engineers, the understanding of the difference. What Is Brittle In Chemistry.
From www.differencebetween.com
Difference Between Ductile and Brittle Deformation Compare the What Is Brittle In Chemistry For engineers, the understanding of the difference between brittle. Brittleness refers to a material’s tendency to fracture or shatter when subjected to stress or impact. In materials science, brittleness is understood as the lack of ductility. Brittleness describes the property of a material that fractures when subjected to stress but has a little tendency to deform before rupture. A brittle. What Is Brittle In Chemistry.
From www.youtube.com
Difference between Ductile and Brittle Materials YouTube What Is Brittle In Chemistry For engineers, the understanding of the difference between brittle. Why are ionic compounds brittle? Since in ceramics the rows cannot slide, the ceramic. What happens when an electric current is passed through a solution of an ionic compound? We have defined brittleness of polymeric materials quantitatively with applications to multiple areas. Why are melting points high for ionic compounds? In. What Is Brittle In Chemistry.
From www.slideserve.com
PPT The World of Materials Linking Physics and Chemistry to What Is Brittle In Chemistry Brittleness refers to a material’s tendency to fracture or shatter when subjected to stress or impact. In metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. A brittle substance has no elasticity and shows little deformation before. Since in ceramics the rows cannot slide, the ceramic. We have defined. What Is Brittle In Chemistry.
From www.sseacademy.com
Ductile and Brittle Materials What Is Brittle In Chemistry Why are melting points high for ionic compounds? Since in ceramics the rows cannot slide, the ceramic. For engineers, the understanding of the difference between brittle. Brittleness refers to a material’s tendency to fracture or shatter when subjected to stress or impact. Why are ionic compounds brittle? In materials science, brittleness is understood as the lack of ductility. We have. What Is Brittle In Chemistry.
From www.instructables.com
Peanut Brittle Chemistry Lab (with Pictures) Instructables What Is Brittle In Chemistry Why are melting points high for ionic compounds? A brittle substance has no elasticity and shows little deformation before. Brittleness describes the property of a material that fractures when subjected to stress but has a little tendency to deform before rupture. Since in ceramics the rows cannot slide, the ceramic. We have defined brittleness of polymeric materials quantitatively with applications. What Is Brittle In Chemistry.
From material-properties.org
What is Brittleness Definition Material Properties What Is Brittle In Chemistry Since in ceramics the rows cannot slide, the ceramic. For engineers, the understanding of the difference between brittle. A brittle substance has no elasticity and shows little deformation before. Why are melting points high for ionic compounds? Brittleness refers to a material’s tendency to fracture or shatter when subjected to stress or impact. Brittleness describes the property of a material. What Is Brittle In Chemistry.