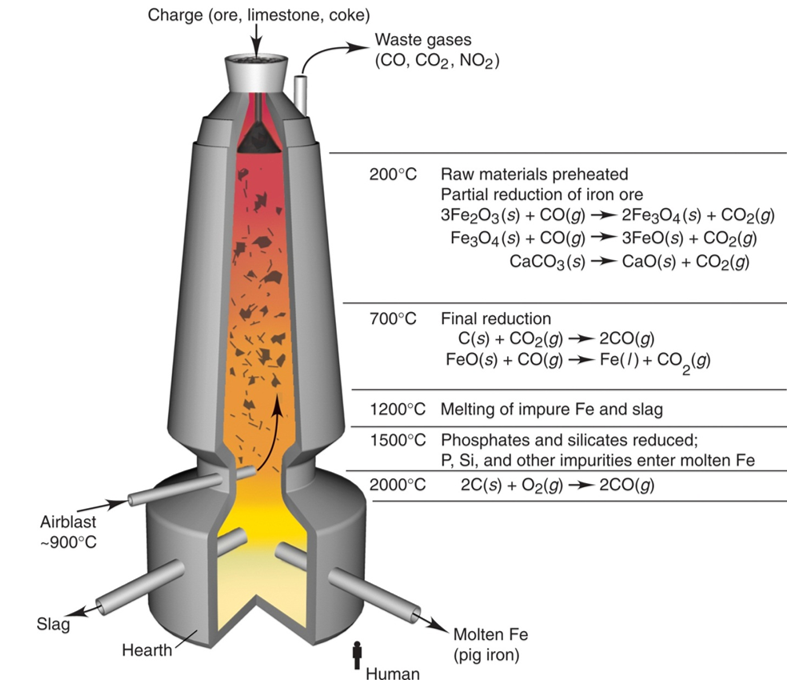
Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace . the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. Slag molten iron air air. metals which are below carbon in the reactivity series are often extracted from their ores by reacting the ores with carbon. (e) iron is extracted from its oxide ore by reduction using carbon in a blast furnace. What happens in the blast furnace? The purpose of a blast furnace is to reduce the concentrated ore chemically to its liquid metal state. it is so hot in the blast furnace that carbon monoxide is the reducing agent which reduces the iron (iii) oxide. the carbon monoxide in the blast furnace reduces the iron(iii) oxide in the hematite ore to produce molten iron. Iron ores contain a large amount of sandy.
from www.w3schools.blog
it is so hot in the blast furnace that carbon monoxide is the reducing agent which reduces the iron (iii) oxide. The purpose of a blast furnace is to reduce the concentrated ore chemically to its liquid metal state. the carbon monoxide in the blast furnace reduces the iron(iii) oxide in the hematite ore to produce molten iron. (e) iron is extracted from its oxide ore by reduction using carbon in a blast furnace. Iron ores contain a large amount of sandy. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. Slag molten iron air air. What happens in the blast furnace? metals which are below carbon in the reactivity series are often extracted from their ores by reacting the ores with carbon. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams.
Iron Extraction W3schools
Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace (e) iron is extracted from its oxide ore by reduction using carbon in a blast furnace. What happens in the blast furnace? revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. it is so hot in the blast furnace that carbon monoxide is the reducing agent which reduces the iron (iii) oxide. Slag molten iron air air. The purpose of a blast furnace is to reduce the concentrated ore chemically to its liquid metal state. (e) iron is extracted from its oxide ore by reduction using carbon in a blast furnace. metals which are below carbon in the reactivity series are often extracted from their ores by reacting the ores with carbon. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. Iron ores contain a large amount of sandy. the carbon monoxide in the blast furnace reduces the iron(iii) oxide in the hematite ore to produce molten iron.
From www.w3schools.blog
Iron Extraction W3schools Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace Iron ores contain a large amount of sandy. the carbon monoxide in the blast furnace reduces the iron(iii) oxide in the hematite ore to produce molten iron. Slag molten iron air air. metals which are below carbon in the reactivity series are often extracted from their ores by reacting the ores with carbon. revision notes on extraction. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From y9mschemistry.blogspot.com
Year 9 Chemistry 2012 Extraction of iron from its ores Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace Slag molten iron air air. Iron ores contain a large amount of sandy. metals which are below carbon in the reactivity series are often extracted from their ores by reacting the ores with carbon. (e) iron is extracted from its oxide ore by reduction using carbon in a blast furnace. What happens in the blast furnace? it. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From www.goodscience.com.au
Extraction of Metals Good Science Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace metals which are below carbon in the reactivity series are often extracted from their ores by reacting the ores with carbon. Slag molten iron air air. Iron ores contain a large amount of sandy. The purpose of a blast furnace is to reduce the concentrated ore chemically to its liquid metal state. the carbon monoxide in the blast. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From byjus.com
How is iron extracted from hematite? Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace What happens in the blast furnace? Slag molten iron air air. the carbon monoxide in the blast furnace reduces the iron(iii) oxide in the hematite ore to produce molten iron. Iron ores contain a large amount of sandy. it is so hot in the blast furnace that carbon monoxide is the reducing agent which reduces the iron (iii). Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From www.mdpi.com
Metals Free FullText Carbon Impact Mitigation of the Iron Ore Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace the carbon monoxide in the blast furnace reduces the iron(iii) oxide in the hematite ore to produce molten iron. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. metals which are below carbon in the reactivity series are often extracted from their ores. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From studyinggcsechem.blogspot.com
IGCSE Chemistry 5.4 Describe and explain the main reactions involved Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. metals which are below carbon in the reactivity series are often extracted from their ores by reacting the ores with carbon. What happens in the blast furnace? the carbon monoxide in the blast furnace. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From app.pandai.org
Extraction of Metals from its Ore Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace What happens in the blast furnace? metals which are below carbon in the reactivity series are often extracted from their ores by reacting the ores with carbon. (e) iron is extracted from its oxide ore by reduction using carbon in a blast furnace. the extraction of iron from its ore is a long and subdued process, that. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From www.dierk-raabe.com
Metallurgical Materials Science and Alloy Design Combining direct and Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. What happens in the blast furnace? the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. the. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From www.savemyexams.co.uk
Extraction of Iron from Hematite (9.3.2) CIE IGCSE Chemistry Revision Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace it is so hot in the blast furnace that carbon monoxide is the reducing agent which reduces the iron (iii) oxide. What happens in the blast furnace? revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. the extraction of iron from its ore. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From www.youtube.com
Extraction Of Iron From Its Oxides YouTube Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace Slag molten iron air air. metals which are below carbon in the reactivity series are often extracted from their ores by reacting the ores with carbon. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. Iron ores contain a large amount of sandy. The. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 2.23C Explain How the Method of Extraction of a Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace (e) iron is extracted from its oxide ore by reduction using carbon in a blast furnace. metals which are below carbon in the reactivity series are often extracted from their ores by reacting the ores with carbon. The purpose of a blast furnace is to reduce the concentrated ore chemically to its liquid metal state. the carbon. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From www.thesciencehive.co.uk
extractionandusesmetalsanswers — the science sauce Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace (e) iron is extracted from its oxide ore by reduction using carbon in a blast furnace. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. metals which are below carbon in the reactivity series are often extracted from their ores by reacting the. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From www.znotes.org
ZNotes For Students. By Students. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. Iron ores contain a large amount of sandy. metals which are below carbon in the reactivity series are often extracted from their ores by reacting the ores with carbon. The purpose. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From spmscience.blog.onlinetuition.com.my
5.4.3 Extraction of Metals from their Ores Using Coke SPM Science Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. metals which are below carbon in the reactivity series are often extracted from their ores by reacting the ores with carbon. the carbon monoxide in the blast furnace reduces the. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From www.metallics.org
Pig iron blast furnace route International Iron Metallics Association Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace the carbon monoxide in the blast furnace reduces the iron(iii) oxide in the hematite ore to produce molten iron. it is so hot in the blast furnace that carbon monoxide is the reducing agent which reduces the iron (iii) oxide. Slag molten iron air air. What happens in the blast furnace? revision notes on extraction of iron. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From www.numerade.com
SOLVED The reduction of iron ore, hematite, takes place in the blast Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace (e) iron is extracted from its oxide ore by reduction using carbon in a blast furnace. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. metals which are below carbon in the reactivity series are often extracted from their ores by reacting the. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From classnotes.org.in
Extraction of Iron Class 12, General Principles and Processes of Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. the carbon monoxide in the blast furnace reduces the iron(iii) oxide in the hematite ore to produce molten iron. Slag molten iron air air. revision notes on extraction of iron. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From y9mschemistry.blogspot.com
Year 9 Chemistry 2012 Extraction of iron from its ores Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. metals which are below carbon in the reactivity series are often extracted from their ores by reacting the ores with carbon. Slag molten iron air air. Iron ores contain a large. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From www.w3schools.blog
Iron Extraction W3schools Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace Slag molten iron air air. the carbon monoxide in the blast furnace reduces the iron(iii) oxide in the hematite ore to produce molten iron. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. (e) iron is extracted from its. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From www.bbc.co.uk
BBC Standard Grade Bitesize Chemistry Reactivity of metals Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace it is so hot in the blast furnace that carbon monoxide is the reducing agent which reduces the iron (iii) oxide. Slag molten iron air air. metals which are below carbon in the reactivity series are often extracted from their ores by reacting the ores with carbon. What happens in the blast furnace? Iron ores contain a large. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From igcseandialchemistry.com
IGCSE Extraction of Metals From Ores Notes IGCSE And IAL Chemistry Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace The purpose of a blast furnace is to reduce the concentrated ore chemically to its liquid metal state. (e) iron is extracted from its oxide ore by reduction using carbon in a blast furnace. Iron ores contain a large amount of sandy. it is so hot in the blast furnace that carbon monoxide is the reducing agent which. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From www.chegg.com
Solved Iron (II) oxide reacts with carbon monoxide to form Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. (e) iron is extracted from its oxide ore by reduction using carbon in a blast furnace. Slag molten iron air air. metals which are below carbon in the reactivity series. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From www.youtube.com
Extraction of Iron Making Iron in the Blast Furnace Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace The purpose of a blast furnace is to reduce the concentrated ore chemically to its liquid metal state. What happens in the blast furnace? Iron ores contain a large amount of sandy. (e) iron is extracted from its oxide ore by reduction using carbon in a blast furnace. Slag molten iron air air. revision notes on extraction of. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From www.onlinemathlearning.com
Extraction of Iron (examples, answers, activities, experiment, videos) Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. it is so hot in the blast. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From www.askiitians.com
Extraction of Iron Study material for IIT JEE askIITians Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace The purpose of a blast furnace is to reduce the concentrated ore chemically to its liquid metal state. Iron ores contain a large amount of sandy. the carbon monoxide in the blast furnace reduces the iron(iii) oxide in the hematite ore to produce molten iron. revision notes on extraction of iron from hematite for the cie igcse chemistry. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From www.vrogue.co
Draw A Neat And Labelled Diagram Of Blast Furnace Cla vrogue.co Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace Slag molten iron air air. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. What happens in the blast furnace? The purpose of a blast furnace is to reduce the concentrated ore chemically to its liquid metal state. the carbon monoxide in the blast. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From www.youtube.com
Extraction of Metals from Ores with basic concepts and Explanation Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace (e) iron is extracted from its oxide ore by reduction using carbon in a blast furnace. Iron ores contain a large amount of sandy. it is so hot in the blast furnace that carbon monoxide is the reducing agent which reduces the iron (iii) oxide. What happens in the blast furnace? The purpose of a blast furnace is. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From superfund.arizona.edu
Copper Mining and Processing Processing Copper Ores Superfund Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace Slag molten iron air air. (e) iron is extracted from its oxide ore by reduction using carbon in a blast furnace. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. Iron ores contain a large amount of sandy. metals. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From cityraven.com
😀 Extraction of iron from its oxides. mining extraction of iron from Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. the carbon monoxide in the blast furnace reduces the iron(iii) oxide in the hematite ore to produce molten iron. The purpose of a blast furnace is to reduce the concentrated ore chemically to its liquid. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From www.revisechemistry.uk
Improving processes and products OCR Gateway C6 revisechemistry.uk Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace the carbon monoxide in the blast furnace reduces the iron(iii) oxide in the hematite ore to produce molten iron. revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. the extraction of iron from its ore is a long and subdued process, that helps. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From cityraven.com
😀 Extraction of iron from its oxides. mining extraction of iron from Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace Iron ores contain a large amount of sandy. What happens in the blast furnace? metals which are below carbon in the reactivity series are often extracted from their ores by reacting the ores with carbon. the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From www.mdpi.com
Materials Free FullText Integrating a TopGas Recycling and CO2 Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace revision notes on extraction of iron from hematite for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. it is so hot in the blast furnace that carbon monoxide is the reducing agent which reduces the iron (iii) oxide. the carbon monoxide in the blast furnace reduces the iron(iii) oxide in the. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From www.tec-science.com
Direct reduced iron process tecscience Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace it is so hot in the blast furnace that carbon monoxide is the reducing agent which reduces the iron (iii) oxide. Iron ores contain a large amount of sandy. What happens in the blast furnace? the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From chem.libretexts.org
23.3 Metallurgy of Iron and Steel Chemistry LibreTexts Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace the carbon monoxide in the blast furnace reduces the iron(iii) oxide in the hematite ore to produce molten iron. The purpose of a blast furnace is to reduce the concentrated ore chemically to its liquid metal state. What happens in the blast furnace? it is so hot in the blast furnace that carbon monoxide is the reducing agent. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.
From www.revisechemistry.uk
Improving processes and products OCR Gateway C6 revisechemistry.uk Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace the extraction of iron from its ore is a long and subdued process, that helps in separating the useful components from the waste materials such as slag. Slag molten iron air air. it is so hot in the blast furnace that carbon monoxide is the reducing agent which reduces the iron (iii) oxide. revision notes on extraction. Iron Is Extracted From Its Oxide Ore By Reduction Using Carbon In A Blast Furnace.