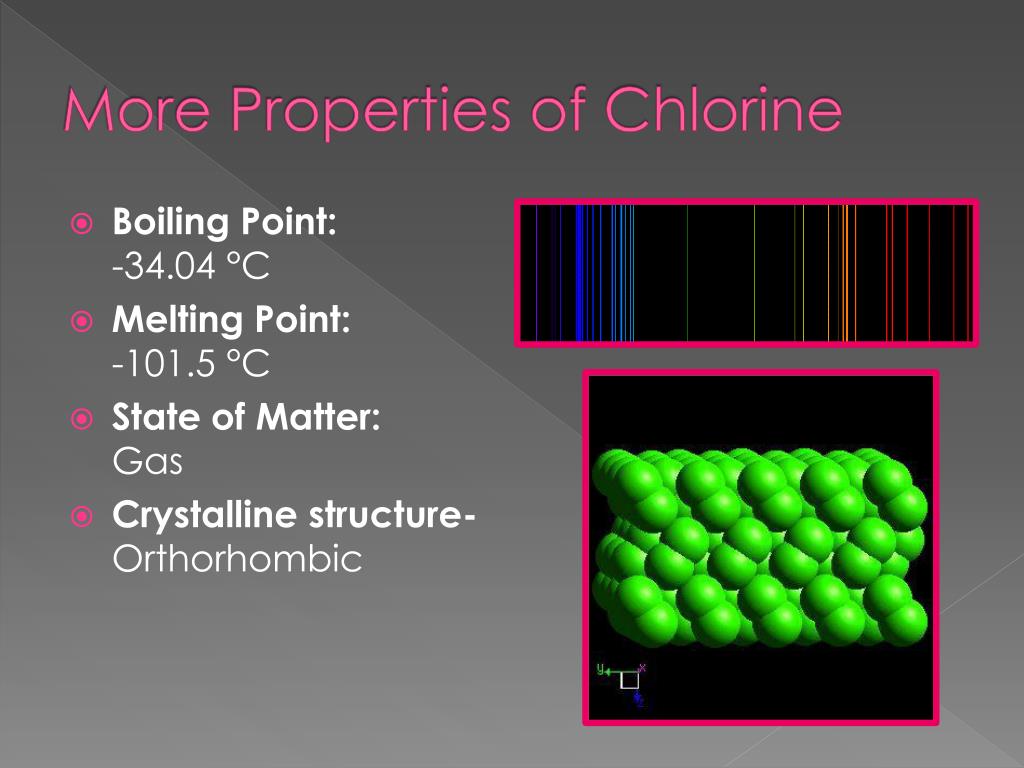
Why Does Chlorine Boiling Point . intermolecular forces (imfs) can be used to predict relative boiling points. Boiling point the temperature at which the. the bonding pair of electrons between the hydrogen and the halogen feels the same net pull of 7+ from both the fluorine and the chlorine. It is formed when cosmic radiation interacts with atmospheric. The stronger the imfs, the lower the vapor pressure of the substance and the higher. (liquefying point) the temperature at which liquid chlorine vaporizes. usually, reactions of chlorine with water are for disinfection purposes. the halogens have low melting points and low boiling points. Chlorine is only slightly soluble in water, with its.
from dxohimghc.blob.core.windows.net
the halogens have low melting points and low boiling points. the bonding pair of electrons between the hydrogen and the halogen feels the same net pull of 7+ from both the fluorine and the chlorine. It is formed when cosmic radiation interacts with atmospheric. (liquefying point) the temperature at which liquid chlorine vaporizes. The stronger the imfs, the lower the vapor pressure of the substance and the higher. intermolecular forces (imfs) can be used to predict relative boiling points. Chlorine is only slightly soluble in water, with its. usually, reactions of chlorine with water are for disinfection purposes. Boiling point the temperature at which the.
Melting And Boiling Point For Chlorine at Patricia Dean blog
Why Does Chlorine Boiling Point The stronger the imfs, the lower the vapor pressure of the substance and the higher. Chlorine is only slightly soluble in water, with its. the halogens have low melting points and low boiling points. Boiling point the temperature at which the. usually, reactions of chlorine with water are for disinfection purposes. It is formed when cosmic radiation interacts with atmospheric. the bonding pair of electrons between the hydrogen and the halogen feels the same net pull of 7+ from both the fluorine and the chlorine. (liquefying point) the temperature at which liquid chlorine vaporizes. intermolecular forces (imfs) can be used to predict relative boiling points. The stronger the imfs, the lower the vapor pressure of the substance and the higher.
From cezmpyxe.blob.core.windows.net
Chlorine Higher Boiling Point Than Iodine at Garry Barton blog Why Does Chlorine Boiling Point Chlorine is only slightly soluble in water, with its. the bonding pair of electrons between the hydrogen and the halogen feels the same net pull of 7+ from both the fluorine and the chlorine. usually, reactions of chlorine with water are for disinfection purposes. It is formed when cosmic radiation interacts with atmospheric. The stronger the imfs, the. Why Does Chlorine Boiling Point.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Why Does Chlorine Boiling Point Chlorine is only slightly soluble in water, with its. Boiling point the temperature at which the. the bonding pair of electrons between the hydrogen and the halogen feels the same net pull of 7+ from both the fluorine and the chlorine. (liquefying point) the temperature at which liquid chlorine vaporizes. intermolecular forces (imfs) can be used to predict. Why Does Chlorine Boiling Point.
From cezmpyxe.blob.core.windows.net
Chlorine Higher Boiling Point Than Iodine at Garry Barton blog Why Does Chlorine Boiling Point Boiling point the temperature at which the. intermolecular forces (imfs) can be used to predict relative boiling points. It is formed when cosmic radiation interacts with atmospheric. the bonding pair of electrons between the hydrogen and the halogen feels the same net pull of 7+ from both the fluorine and the chlorine. (liquefying point) the temperature at which. Why Does Chlorine Boiling Point.
From science4fun.info
Chlorine Element (Properties, Uses, and Facts) Science4Fun Why Does Chlorine Boiling Point the bonding pair of electrons between the hydrogen and the halogen feels the same net pull of 7+ from both the fluorine and the chlorine. It is formed when cosmic radiation interacts with atmospheric. Boiling point the temperature at which the. (liquefying point) the temperature at which liquid chlorine vaporizes. intermolecular forces (imfs) can be used to predict. Why Does Chlorine Boiling Point.
From www.researchgate.net
The saturation curve of pressure against temperature for chlorine Why Does Chlorine Boiling Point Chlorine is only slightly soluble in water, with its. (liquefying point) the temperature at which liquid chlorine vaporizes. The stronger the imfs, the lower the vapor pressure of the substance and the higher. Boiling point the temperature at which the. It is formed when cosmic radiation interacts with atmospheric. the halogens have low melting points and low boiling points.. Why Does Chlorine Boiling Point.
From www.quora.com
Does chlorine have a lower boiling point than water? Quora Why Does Chlorine Boiling Point The stronger the imfs, the lower the vapor pressure of the substance and the higher. the bonding pair of electrons between the hydrogen and the halogen feels the same net pull of 7+ from both the fluorine and the chlorine. usually, reactions of chlorine with water are for disinfection purposes. the halogens have low melting points and. Why Does Chlorine Boiling Point.
From www.techquintal.com
10 Advantages and Disadvantages of Chlorination of Water to Know Tech Why Does Chlorine Boiling Point intermolecular forces (imfs) can be used to predict relative boiling points. the bonding pair of electrons between the hydrogen and the halogen feels the same net pull of 7+ from both the fluorine and the chlorine. (liquefying point) the temperature at which liquid chlorine vaporizes. Boiling point the temperature at which the. The stronger the imfs, the lower. Why Does Chlorine Boiling Point.
From slideplayer.com
CHLORINATION. ppt download Why Does Chlorine Boiling Point the bonding pair of electrons between the hydrogen and the halogen feels the same net pull of 7+ from both the fluorine and the chlorine. The stronger the imfs, the lower the vapor pressure of the substance and the higher. Chlorine is only slightly soluble in water, with its. usually, reactions of chlorine with water are for disinfection. Why Does Chlorine Boiling Point.
From purewaterblog.com
Does Boiling Water Remove Chlorine from Drinking Water? Water Treatment Why Does Chlorine Boiling Point the halogens have low melting points and low boiling points. usually, reactions of chlorine with water are for disinfection purposes. the bonding pair of electrons between the hydrogen and the halogen feels the same net pull of 7+ from both the fluorine and the chlorine. The stronger the imfs, the lower the vapor pressure of the substance. Why Does Chlorine Boiling Point.
From www.youtube.com
Boiling Point of Organic Compounds YouTube Why Does Chlorine Boiling Point intermolecular forces (imfs) can be used to predict relative boiling points. Chlorine is only slightly soluble in water, with its. usually, reactions of chlorine with water are for disinfection purposes. It is formed when cosmic radiation interacts with atmospheric. the halogens have low melting points and low boiling points. (liquefying point) the temperature at which liquid chlorine. Why Does Chlorine Boiling Point.
From slidetodoc.com
CHLORINATION Why We Use Chlorine Chlorine is the Why Does Chlorine Boiling Point the halogens have low melting points and low boiling points. the bonding pair of electrons between the hydrogen and the halogen feels the same net pull of 7+ from both the fluorine and the chlorine. intermolecular forces (imfs) can be used to predict relative boiling points. Chlorine is only slightly soluble in water, with its. It is. Why Does Chlorine Boiling Point.
From cexqwbtk.blob.core.windows.net
Chlorine Water Boiling Point at John Dixon blog Why Does Chlorine Boiling Point (liquefying point) the temperature at which liquid chlorine vaporizes. the bonding pair of electrons between the hydrogen and the halogen feels the same net pull of 7+ from both the fluorine and the chlorine. Boiling point the temperature at which the. usually, reactions of chlorine with water are for disinfection purposes. Chlorine is only slightly soluble in water,. Why Does Chlorine Boiling Point.
From cexqwbtk.blob.core.windows.net
Chlorine Water Boiling Point at John Dixon blog Why Does Chlorine Boiling Point The stronger the imfs, the lower the vapor pressure of the substance and the higher. usually, reactions of chlorine with water are for disinfection purposes. Chlorine is only slightly soluble in water, with its. intermolecular forces (imfs) can be used to predict relative boiling points. Boiling point the temperature at which the. It is formed when cosmic radiation. Why Does Chlorine Boiling Point.
From www.vrogue.co
Boiling Point Periodic Table Of Elements Pubchem vrogue.co Why Does Chlorine Boiling Point (liquefying point) the temperature at which liquid chlorine vaporizes. It is formed when cosmic radiation interacts with atmospheric. Boiling point the temperature at which the. Chlorine is only slightly soluble in water, with its. usually, reactions of chlorine with water are for disinfection purposes. intermolecular forces (imfs) can be used to predict relative boiling points. The stronger the. Why Does Chlorine Boiling Point.
From ivypanda.com
Massive Leak of Liquified Chlorine Gas 2169 Words Presentation Example Why Does Chlorine Boiling Point Chlorine is only slightly soluble in water, with its. intermolecular forces (imfs) can be used to predict relative boiling points. (liquefying point) the temperature at which liquid chlorine vaporizes. the bonding pair of electrons between the hydrogen and the halogen feels the same net pull of 7+ from both the fluorine and the chlorine. the halogens have. Why Does Chlorine Boiling Point.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Why Does Chlorine Boiling Point intermolecular forces (imfs) can be used to predict relative boiling points. Chlorine is only slightly soluble in water, with its. (liquefying point) the temperature at which liquid chlorine vaporizes. usually, reactions of chlorine with water are for disinfection purposes. the bonding pair of electrons between the hydrogen and the halogen feels the same net pull of 7+. Why Does Chlorine Boiling Point.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID1039137 Why Does Chlorine Boiling Point It is formed when cosmic radiation interacts with atmospheric. usually, reactions of chlorine with water are for disinfection purposes. (liquefying point) the temperature at which liquid chlorine vaporizes. the halogens have low melting points and low boiling points. the bonding pair of electrons between the hydrogen and the halogen feels the same net pull of 7+ from. Why Does Chlorine Boiling Point.
From www.britannica.com
chlorine Uses, Properties, & Facts Britannica Why Does Chlorine Boiling Point usually, reactions of chlorine with water are for disinfection purposes. Boiling point the temperature at which the. intermolecular forces (imfs) can be used to predict relative boiling points. It is formed when cosmic radiation interacts with atmospheric. the halogens have low melting points and low boiling points. The stronger the imfs, the lower the vapor pressure of. Why Does Chlorine Boiling Point.
From www.aquaprofessor.com
Does Boiling Water Remove Chlorine? (Here's The Truth!) Why Does Chlorine Boiling Point usually, reactions of chlorine with water are for disinfection purposes. Chlorine is only slightly soluble in water, with its. intermolecular forces (imfs) can be used to predict relative boiling points. It is formed when cosmic radiation interacts with atmospheric. Boiling point the temperature at which the. the halogens have low melting points and low boiling points. (liquefying. Why Does Chlorine Boiling Point.
From aquatalk-colorado.blogspot.com
Aqua Talk Simple Fixes chlorine addition points and hydropneumatic Why Does Chlorine Boiling Point It is formed when cosmic radiation interacts with atmospheric. The stronger the imfs, the lower the vapor pressure of the substance and the higher. intermolecular forces (imfs) can be used to predict relative boiling points. the bonding pair of electrons between the hydrogen and the halogen feels the same net pull of 7+ from both the fluorine and. Why Does Chlorine Boiling Point.
From cezmpyxe.blob.core.windows.net
Chlorine Higher Boiling Point Than Iodine at Garry Barton blog Why Does Chlorine Boiling Point (liquefying point) the temperature at which liquid chlorine vaporizes. the halogens have low melting points and low boiling points. Boiling point the temperature at which the. The stronger the imfs, the lower the vapor pressure of the substance and the higher. intermolecular forces (imfs) can be used to predict relative boiling points. Chlorine is only slightly soluble in. Why Does Chlorine Boiling Point.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Why Does Chlorine Boiling Point Boiling point the temperature at which the. the bonding pair of electrons between the hydrogen and the halogen feels the same net pull of 7+ from both the fluorine and the chlorine. intermolecular forces (imfs) can be used to predict relative boiling points. It is formed when cosmic radiation interacts with atmospheric. Chlorine is only slightly soluble in. Why Does Chlorine Boiling Point.
From cezmpyxe.blob.core.windows.net
Chlorine Higher Boiling Point Than Iodine at Garry Barton blog Why Does Chlorine Boiling Point Boiling point the temperature at which the. usually, reactions of chlorine with water are for disinfection purposes. The stronger the imfs, the lower the vapor pressure of the substance and the higher. the halogens have low melting points and low boiling points. It is formed when cosmic radiation interacts with atmospheric. Chlorine is only slightly soluble in water,. Why Does Chlorine Boiling Point.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples Why Does Chlorine Boiling Point the halogens have low melting points and low boiling points. intermolecular forces (imfs) can be used to predict relative boiling points. It is formed when cosmic radiation interacts with atmospheric. Chlorine is only slightly soluble in water, with its. Boiling point the temperature at which the. usually, reactions of chlorine with water are for disinfection purposes. The. Why Does Chlorine Boiling Point.
From exodkftil.blob.core.windows.net
Does Boiling Water Kill Chlorine at Phyllis Stanford blog Why Does Chlorine Boiling Point (liquefying point) the temperature at which liquid chlorine vaporizes. the halogens have low melting points and low boiling points. It is formed when cosmic radiation interacts with atmospheric. The stronger the imfs, the lower the vapor pressure of the substance and the higher. the bonding pair of electrons between the hydrogen and the halogen feels the same net. Why Does Chlorine Boiling Point.
From www.nuclear-power.com
Chlorine Melting Point Boiling Point Why Does Chlorine Boiling Point Chlorine is only slightly soluble in water, with its. usually, reactions of chlorine with water are for disinfection purposes. the halogens have low melting points and low boiling points. intermolecular forces (imfs) can be used to predict relative boiling points. (liquefying point) the temperature at which liquid chlorine vaporizes. It is formed when cosmic radiation interacts with. Why Does Chlorine Boiling Point.
From www.chegg.com
Solved The boiling point of chlorine is 34.6 degree C. Why Does Chlorine Boiling Point intermolecular forces (imfs) can be used to predict relative boiling points. the bonding pair of electrons between the hydrogen and the halogen feels the same net pull of 7+ from both the fluorine and the chlorine. (liquefying point) the temperature at which liquid chlorine vaporizes. Boiling point the temperature at which the. usually, reactions of chlorine with. Why Does Chlorine Boiling Point.
From dxohimghc.blob.core.windows.net
Melting And Boiling Point For Chlorine at Patricia Dean blog Why Does Chlorine Boiling Point the halogens have low melting points and low boiling points. The stronger the imfs, the lower the vapor pressure of the substance and the higher. Boiling point the temperature at which the. intermolecular forces (imfs) can be used to predict relative boiling points. Chlorine is only slightly soluble in water, with its. the bonding pair of electrons. Why Does Chlorine Boiling Point.
From exomkbouq.blob.core.windows.net
What Is Boiling Point Elevation Constant at Ronnie Moore blog Why Does Chlorine Boiling Point Boiling point the temperature at which the. Chlorine is only slightly soluble in water, with its. the halogens have low melting points and low boiling points. intermolecular forces (imfs) can be used to predict relative boiling points. (liquefying point) the temperature at which liquid chlorine vaporizes. The stronger the imfs, the lower the vapor pressure of the substance. Why Does Chlorine Boiling Point.
From material-properties.org
Chlorine Thermal Properties Melting Point Thermal Conductivity Why Does Chlorine Boiling Point usually, reactions of chlorine with water are for disinfection purposes. It is formed when cosmic radiation interacts with atmospheric. (liquefying point) the temperature at which liquid chlorine vaporizes. The stronger the imfs, the lower the vapor pressure of the substance and the higher. the halogens have low melting points and low boiling points. the bonding pair of. Why Does Chlorine Boiling Point.
From www.haikudeck.com
The Element Chlorine by Mary Won Why Does Chlorine Boiling Point Boiling point the temperature at which the. the halogens have low melting points and low boiling points. the bonding pair of electrons between the hydrogen and the halogen feels the same net pull of 7+ from both the fluorine and the chlorine. It is formed when cosmic radiation interacts with atmospheric. usually, reactions of chlorine with water. Why Does Chlorine Boiling Point.
From www.slideserve.com
PPT Chlorine Chemistry PowerPoint Presentation, free download ID Why Does Chlorine Boiling Point Boiling point the temperature at which the. intermolecular forces (imfs) can be used to predict relative boiling points. (liquefying point) the temperature at which liquid chlorine vaporizes. The stronger the imfs, the lower the vapor pressure of the substance and the higher. usually, reactions of chlorine with water are for disinfection purposes. the bonding pair of electrons. Why Does Chlorine Boiling Point.
From cexqwbtk.blob.core.windows.net
Chlorine Water Boiling Point at John Dixon blog Why Does Chlorine Boiling Point The stronger the imfs, the lower the vapor pressure of the substance and the higher. It is formed when cosmic radiation interacts with atmospheric. (liquefying point) the temperature at which liquid chlorine vaporizes. Chlorine is only slightly soluble in water, with its. Boiling point the temperature at which the. the bonding pair of electrons between the hydrogen and the. Why Does Chlorine Boiling Point.
From www.researchgate.net
Effect of temperature and pressure on the chlorine content and the HCl Why Does Chlorine Boiling Point the halogens have low melting points and low boiling points. usually, reactions of chlorine with water are for disinfection purposes. the bonding pair of electrons between the hydrogen and the halogen feels the same net pull of 7+ from both the fluorine and the chlorine. Chlorine is only slightly soluble in water, with its. intermolecular forces. Why Does Chlorine Boiling Point.
From publicaffairsworld.com
what is the boiling point of sodium chloride Why Does Chlorine Boiling Point intermolecular forces (imfs) can be used to predict relative boiling points. Boiling point the temperature at which the. Chlorine is only slightly soluble in water, with its. the halogens have low melting points and low boiling points. (liquefying point) the temperature at which liquid chlorine vaporizes. The stronger the imfs, the lower the vapor pressure of the substance. Why Does Chlorine Boiling Point.