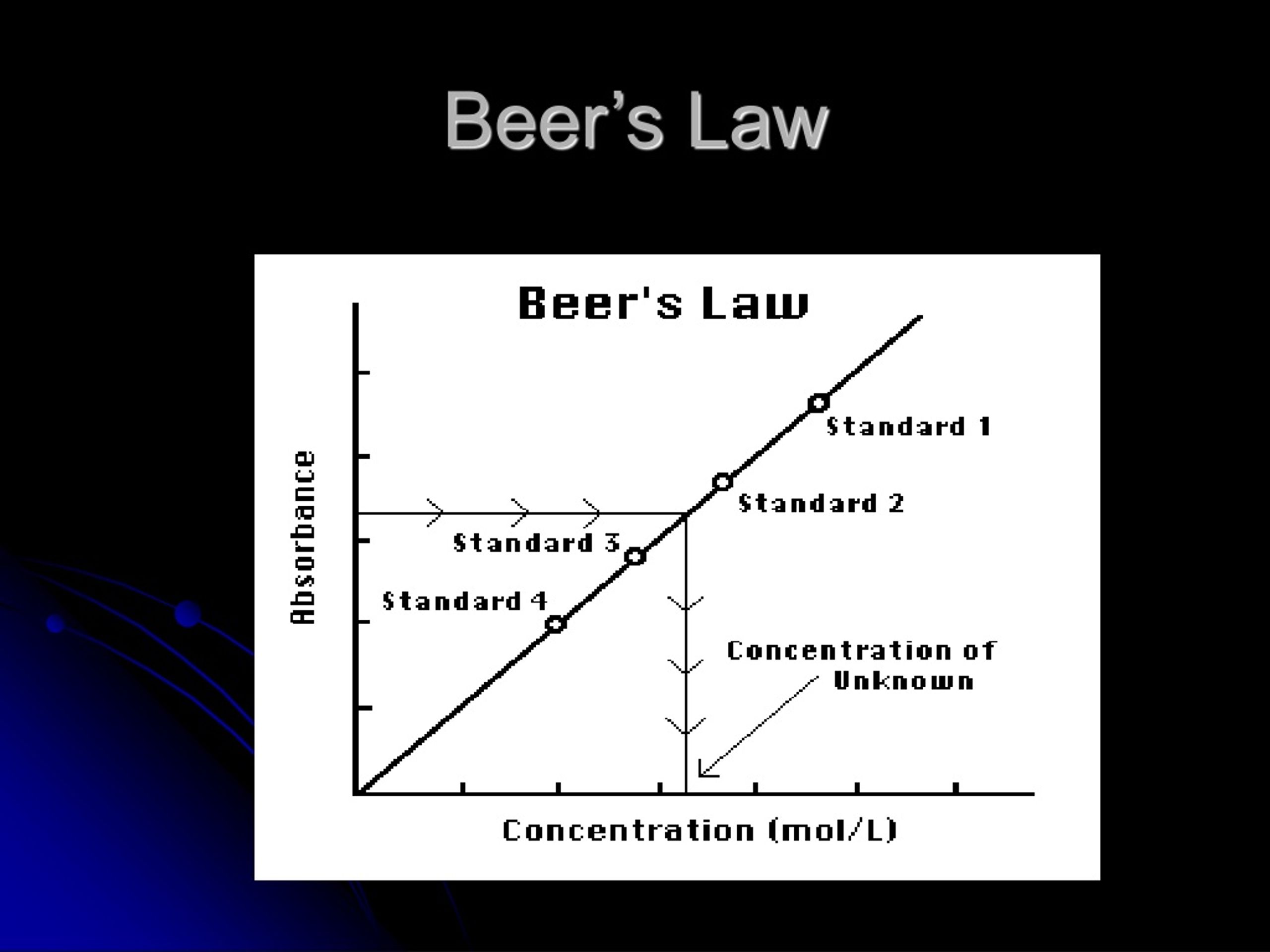
Beer's Law Is Applicable Only To . It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. In other words, a solution. However, for simplicity’s sake, it is often simply called beer’s law. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration.
from www.slideserve.com
The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. However, for simplicity’s sake, it is often simply called beer’s law. In other words, a solution. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light.
PPT Review of Basic Concepts, Molarity, Solutions, Dilutions and Beer
Beer's Law Is Applicable Only To In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In other words, a solution. However, for simplicity’s sake, it is often simply called beer’s law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation Beer's Law Is Applicable Only To It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. Since the. Beer's Law Is Applicable Only To.
From studylib.net
Beer`s Law Beer's Law Is Applicable Only To In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. Since the concentration, path length and molar absorptivity are all directly. Beer's Law Is Applicable Only To.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law Is Applicable Only To The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the. Beer's Law Is Applicable Only To.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law Is Applicable Only To In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. However, for simplicity’s sake, it is often simply called beer’s law. The absorbance of a solution is directly proportional. Beer's Law Is Applicable Only To.
From www.youtube.com
Beer Lambert Law simplest explanation animation YouTube Beer's Law Is Applicable Only To However, for simplicity’s sake, it is often simply called beer’s law. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The absorbance of a solution is directly proportional to the path. Beer's Law Is Applicable Only To.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Is Applicable Only To However, for simplicity’s sake, it is often simply called beer’s law. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In other words, a solution. In spectroscopy, beer’s law states that. Beer's Law Is Applicable Only To.
From www.youtube.com
Ch 17 7 Beer's Law is Additive for All Components YouTube Beer's Law Is Applicable Only To Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. Beer's Law Is Applicable Only To.
From www.youtube.com
Absorption spectroscopy (BeerLambert law) presented by PhD Emil Beer's Law Is Applicable Only To In other words, a solution. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance,. Beer's Law Is Applicable Only To.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Is Applicable Only To Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. However, for simplicity’s sake, it is often simply called beer’s law. In other words, a solution. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The absorbance of a solution is directly proportional. Beer's Law Is Applicable Only To.
From www.slideserve.com
PPT Beers Law for a Single Component Sample PowerPoint Presentation Beer's Law Is Applicable Only To In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. However,. Beer's Law Is Applicable Only To.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Is Applicable Only To Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. In other words, a solution. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length. Beer's Law Is Applicable Only To.
From www.youtube.com
BeerLambert law in easy way YouTube Beer's Law Is Applicable Only To In other words, a solution. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. However, for simplicity’s sake, it is often simply called beer’s law. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In most experiments, molar absorptivity (ε). Beer's Law Is Applicable Only To.
From www.slideserve.com
PPT Beer’s Law and Spectrophotometry PowerPoint Presentation, free Beer's Law Is Applicable Only To In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. Since the. Beer's Law Is Applicable Only To.
From brainly.in
Beer lambert law formula Brainly.in Beer's Law Is Applicable Only To Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In other words, a solution. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The. Beer's Law Is Applicable Only To.
From lukewarmtakes.net
Spectrophotometry and Beer's Law Lukewarm Takes Beer's Law Is Applicable Only To In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution.. Beer's Law Is Applicable Only To.
From www.slideshare.net
Beers law Beer's Law Is Applicable Only To Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. However, for simplicity’s sake, it is often simply called beer’s law. In most experiments, molar. Beer's Law Is Applicable Only To.
From www.chegg.com
Solved A Beer's Law plot is shown here along with the Beer's Law Is Applicable Only To However, for simplicity’s sake, it is often simply called beer’s law. In other words, a solution. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the. Beer's Law Is Applicable Only To.
From www.youtube.com
Beer's Law Explanation and Example YouTube Beer's Law Is Applicable Only To In other words, a solution. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The absorbance of a solution is directly proportional to the path length. Beer's Law Is Applicable Only To.
From www.slideserve.com
PPT Beer’s Law and Spectrophotometry PowerPoint Presentation ID211563 Beer's Law Is Applicable Only To It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the. Beer's Law Is Applicable Only To.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer's Law Is Applicable Only To The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to. Beer's Law Is Applicable Only To.
From www.scribd.com
Beer S Law Plot of KMnO4 PDF PDF Beer's Law Is Applicable Only To The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In other words, a solution. However, for simplicity’s sake, it is often simply called beer’s law. Since the. Beer's Law Is Applicable Only To.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer's Law Is Applicable Only To It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. However, for simplicity’s sake, it is often simply called beer’s law. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. In spectroscopy, beer’s law states that the. Beer's Law Is Applicable Only To.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Is Applicable Only To In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Since. Beer's Law Is Applicable Only To.
From www.slideserve.com
PPT Review of Basic Concepts, Molarity, Solutions, Dilutions and Beer Beer's Law Is Applicable Only To In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. In other words, a solution. However, for simplicity’s sake, it is often simply called beer’s law. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Since the concentration,. Beer's Law Is Applicable Only To.
From www.slideserve.com
PPT Beer’s Law and Spectrophotometry PowerPoint Presentation, free Beer's Law Is Applicable Only To Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In other words, a solution. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. However, for simplicity’s sake, it is often simply called beer’s. Beer's Law Is Applicable Only To.
From rrennenextanner.blogspot.com
Beer Lambert Law Ppt rrennenexTanner Beer's Law Is Applicable Only To In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the. Beer's Law Is Applicable Only To.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Is Applicable Only To In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. Since the concentration, path length and molar absorptivity are all directly. Beer's Law Is Applicable Only To.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer's Law Is Applicable Only To It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. However, for simplicity’s sake, it is often simply called beer’s law. In other words, a solution. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. Since the. Beer's Law Is Applicable Only To.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW Beer's Law Is Applicable Only To The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. However, for simplicity’s sake, it is often simply called beer’s law. In spectroscopy, beer’s law states that the absorption of. Beer's Law Is Applicable Only To.
From www.slideserve.com
PPT Deviations to Beers Law PowerPoint Presentation, free download Beer's Law Is Applicable Only To In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. It provides a mathematical relationship between the substance’s concentration in a. Beer's Law Is Applicable Only To.
From www.youtube.com
Beer's Law Concept, Calculations, & Lab YouTube Beer's Law Is Applicable Only To In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. However, for simplicity’s sake, it is often simply called beer’s law. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution.. Beer's Law Is Applicable Only To.
From goformative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Formative Beer's Law Is Applicable Only To In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In other words, a solution. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The absorbance of a solution is directly proportional to the. Beer's Law Is Applicable Only To.
From www.youtube.com
Derivation of Beer Lambert Law YouTube Beer's Law Is Applicable Only To In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. However, for simplicity’s sake, it is often simply called beer’s law. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance,. Beer's Law Is Applicable Only To.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law Is Applicable Only To Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In other words, a solution. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. In most experiments, molar absorptivity (ε) and the length (b) are. Beer's Law Is Applicable Only To.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law Is Applicable Only To In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. However, for simplicity’s sake, it is often simply called beer’s law. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance,. Beer's Law Is Applicable Only To.