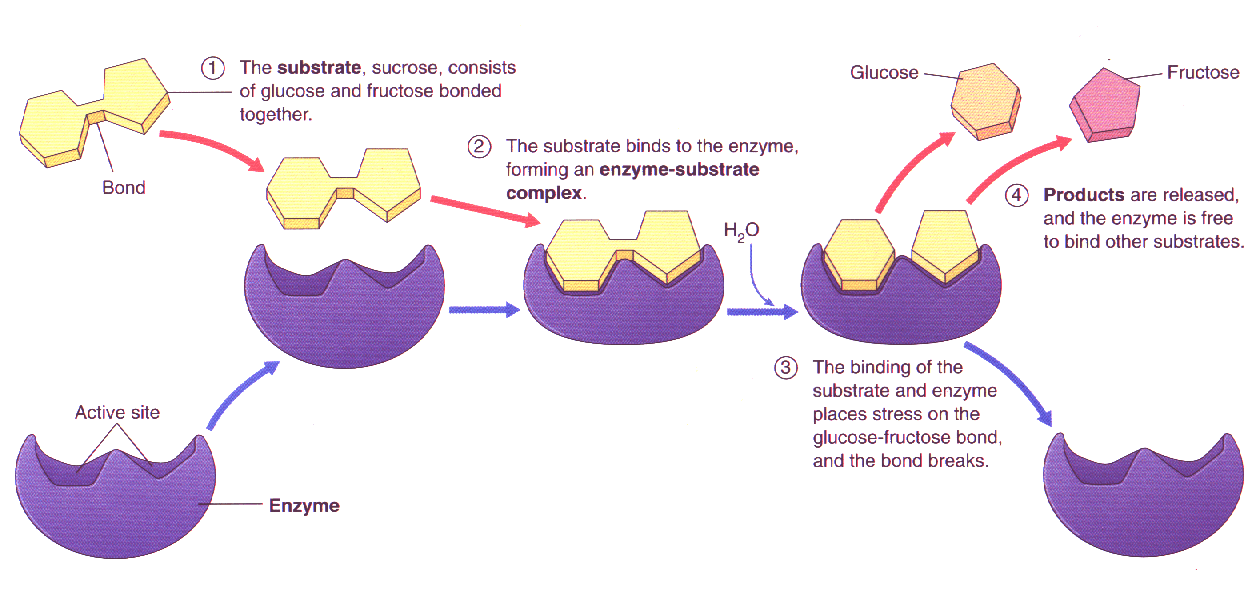
Explain How Enzymes Lower Activation Energy . The activation energy (ea) is actually the energy required to form the transition state, so enzymes lower the ea by stabilising the transition state,. This means their shape (as well as the shape of the active site of an enzyme) is determined by the complex. Enzymes can lower the activation energy of a chemical reaction in three ways. The increased rate is the same in both. Enzymes work by lowering the activation energy needed to start biochemical reactions. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. The activities of enzymes depend on the temperature, ionic conditions, and the ph of the surroundings. One of the ways the activation energy is lowered is having the. The way enzymes lower activation energy is by providing an alternative reaction pathway with a lower activation energy.
from linfeibioblog.blogspot.com
The activation energy (ea) is actually the energy required to form the transition state, so enzymes lower the ea by stabilising the transition state,. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. Enzymes can lower the activation energy of a chemical reaction in three ways. The increased rate is the same in both. One of the ways the activation energy is lowered is having the. This means their shape (as well as the shape of the active site of an enzyme) is determined by the complex. The activities of enzymes depend on the temperature, ionic conditions, and the ph of the surroundings. The way enzymes lower activation energy is by providing an alternative reaction pathway with a lower activation energy. Enzymes work by lowering the activation energy needed to start biochemical reactions.
Linfei's Bio Blog Enzymes
Explain How Enzymes Lower Activation Energy The activation energy (ea) is actually the energy required to form the transition state, so enzymes lower the ea by stabilising the transition state,. The increased rate is the same in both. One of the ways the activation energy is lowered is having the. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. The activities of enzymes depend on the temperature, ionic conditions, and the ph of the surroundings. Enzymes can lower the activation energy of a chemical reaction in three ways. Enzymes work by lowering the activation energy needed to start biochemical reactions. The way enzymes lower activation energy is by providing an alternative reaction pathway with a lower activation energy. This means their shape (as well as the shape of the active site of an enzyme) is determined by the complex. The activation energy (ea) is actually the energy required to form the transition state, so enzymes lower the ea by stabilising the transition state,.
From www.youtube.com
Enzyme Basics & How Do They Lower Activation Energy? YouTube Explain How Enzymes Lower Activation Energy The activation energy (ea) is actually the energy required to form the transition state, so enzymes lower the ea by stabilising the transition state,. The way enzymes lower activation energy is by providing an alternative reaction pathway with a lower activation energy. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. The activities. Explain How Enzymes Lower Activation Energy.
From slideplayer.com
Enzymes Biological catalysts ppt download Explain How Enzymes Lower Activation Energy The way enzymes lower activation energy is by providing an alternative reaction pathway with a lower activation energy. Enzymes work by lowering the activation energy needed to start biochemical reactions. Enzymes can lower the activation energy of a chemical reaction in three ways. The activation energy (ea) is actually the energy required to form the transition state, so enzymes lower. Explain How Enzymes Lower Activation Energy.
From present5.com
Enzyme Structure classification and mechanism of action Explain How Enzymes Lower Activation Energy Enzymes work by lowering the activation energy needed to start biochemical reactions. The increased rate is the same in both. This means their shape (as well as the shape of the active site of an enzyme) is determined by the complex. Enzymes can lower the activation energy of a chemical reaction in three ways. Enzymes (and other catalysts) act by. Explain How Enzymes Lower Activation Energy.
From www.expii.com
Enzyme Inhibition — Overview & Types Expii Explain How Enzymes Lower Activation Energy One of the ways the activation energy is lowered is having the. Enzymes can lower the activation energy of a chemical reaction in three ways. The way enzymes lower activation energy is by providing an alternative reaction pathway with a lower activation energy. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. The. Explain How Enzymes Lower Activation Energy.
From www.youtube.com
Enzymes & Activation Energy YouTube Explain How Enzymes Lower Activation Energy The increased rate is the same in both. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. This means their shape (as well as the shape of the active site of an enzyme) is determined by the complex. One of the ways the activation energy is lowered is having the. Enzymes work by. Explain How Enzymes Lower Activation Energy.
From brainly.com
12.) Using graph 1, explain how enzymes work. Include the term Explain How Enzymes Lower Activation Energy This means their shape (as well as the shape of the active site of an enzyme) is determined by the complex. The way enzymes lower activation energy is by providing an alternative reaction pathway with a lower activation energy. Enzymes work by lowering the activation energy needed to start biochemical reactions. One of the ways the activation energy is lowered. Explain How Enzymes Lower Activation Energy.
From studylib.net
Enzymes Lower Activation Energy Explain How Enzymes Lower Activation Energy One of the ways the activation energy is lowered is having the. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. The increased rate is the same in both. The activities of enzymes depend on the temperature, ionic conditions, and the ph of the surroundings. Enzymes work by lowering the activation energy needed. Explain How Enzymes Lower Activation Energy.
From www.onlinebiologynotes.com
Enzymes Properties and Mechanism of enzyme action Online Biology Notes Explain How Enzymes Lower Activation Energy The activation energy (ea) is actually the energy required to form the transition state, so enzymes lower the ea by stabilising the transition state,. The way enzymes lower activation energy is by providing an alternative reaction pathway with a lower activation energy. The increased rate is the same in both. The activities of enzymes depend on the temperature, ionic conditions,. Explain How Enzymes Lower Activation Energy.
From www.pinterest.com
Pin on Explain How Enzymes Lower Activation Energy The increased rate is the same in both. The activities of enzymes depend on the temperature, ionic conditions, and the ph of the surroundings. The way enzymes lower activation energy is by providing an alternative reaction pathway with a lower activation energy. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. This means. Explain How Enzymes Lower Activation Energy.
From animalia-life.club
Enzyme Activation Energy Graph Explain How Enzymes Lower Activation Energy This means their shape (as well as the shape of the active site of an enzyme) is determined by the complex. Enzymes work by lowering the activation energy needed to start biochemical reactions. The activities of enzymes depend on the temperature, ionic conditions, and the ph of the surroundings. Enzymes can lower the activation energy of a chemical reaction in. Explain How Enzymes Lower Activation Energy.
From zhtutorials.com
Factors Affecting Enzyme Activity Enzymes Ep 2 Zoë Huggett Tutorials Explain How Enzymes Lower Activation Energy The activation energy (ea) is actually the energy required to form the transition state, so enzymes lower the ea by stabilising the transition state,. One of the ways the activation energy is lowered is having the. Enzymes can lower the activation energy of a chemical reaction in three ways. The activities of enzymes depend on the temperature, ionic conditions, and. Explain How Enzymes Lower Activation Energy.
From www.thoughtco.com
Structure and Function of an Enzyme Explain How Enzymes Lower Activation Energy The increased rate is the same in both. The activities of enzymes depend on the temperature, ionic conditions, and the ph of the surroundings. Enzymes work by lowering the activation energy needed to start biochemical reactions. The activation energy (ea) is actually the energy required to form the transition state, so enzymes lower the ea by stabilising the transition state,.. Explain How Enzymes Lower Activation Energy.
From www2.nau.edu
Enzymes and Reaction Rates Explain How Enzymes Lower Activation Energy The activities of enzymes depend on the temperature, ionic conditions, and the ph of the surroundings. The way enzymes lower activation energy is by providing an alternative reaction pathway with a lower activation energy. Enzymes work by lowering the activation energy needed to start biochemical reactions. The activation energy (ea) is actually the energy required to form the transition state,. Explain How Enzymes Lower Activation Energy.
From inzymo.blogspot.com
Enzymes the cellular machinery Explain How Enzymes Lower Activation Energy This means their shape (as well as the shape of the active site of an enzyme) is determined by the complex. The activities of enzymes depend on the temperature, ionic conditions, and the ph of the surroundings. The way enzymes lower activation energy is by providing an alternative reaction pathway with a lower activation energy. The activation energy (ea) is. Explain How Enzymes Lower Activation Energy.
From www.slideserve.com
PPT 7.6 Enzymes (AHL) PowerPoint Presentation, free download ID2742506 Explain How Enzymes Lower Activation Energy Enzymes can lower the activation energy of a chemical reaction in three ways. The activation energy (ea) is actually the energy required to form the transition state, so enzymes lower the ea by stabilising the transition state,. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. The activities of enzymes depend on the. Explain How Enzymes Lower Activation Energy.
From telegra.ph
Describe how enzymes lower activation energy Telegraph Explain How Enzymes Lower Activation Energy Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. This means their shape (as well as the shape of the active site of an enzyme) is determined by the complex. The activities of enzymes depend on the temperature, ionic conditions, and the ph of the surroundings. One of the ways the activation energy. Explain How Enzymes Lower Activation Energy.
From www.pinterest.com
Image from Explain How Enzymes Lower Activation Energy Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. The increased rate is the same in both. The way enzymes lower activation energy is by providing an alternative reaction pathway with a lower activation energy. The activities of enzymes depend on the temperature, ionic conditions, and the ph of the surroundings. One of. Explain How Enzymes Lower Activation Energy.
From telepathhero.blogspot.com
TelepathHero november 2016 Explain How Enzymes Lower Activation Energy Enzymes work by lowering the activation energy needed to start biochemical reactions. The increased rate is the same in both. One of the ways the activation energy is lowered is having the. The activation energy (ea) is actually the energy required to form the transition state, so enzymes lower the ea by stabilising the transition state,. The way enzymes lower. Explain How Enzymes Lower Activation Energy.
From thebiologs.blogspot.com
The BioLogs Ezymes CSEC Explain How Enzymes Lower Activation Energy The activation energy (ea) is actually the energy required to form the transition state, so enzymes lower the ea by stabilising the transition state,. This means their shape (as well as the shape of the active site of an enzyme) is determined by the complex. The increased rate is the same in both. Enzymes work by lowering the activation energy. Explain How Enzymes Lower Activation Energy.
From dokumen.tips
(PPT) Enzymes 5. Objectives Explain how enzymes affect activation Explain How Enzymes Lower Activation Energy The way enzymes lower activation energy is by providing an alternative reaction pathway with a lower activation energy. The activities of enzymes depend on the temperature, ionic conditions, and the ph of the surroundings. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. Enzymes can lower the activation energy of a chemical reaction. Explain How Enzymes Lower Activation Energy.
From www.linstitute.net
IB DP Biology HL复习笔记8.1.1 Metabolic Pathways翰林国际教育 Explain How Enzymes Lower Activation Energy The activation energy (ea) is actually the energy required to form the transition state, so enzymes lower the ea by stabilising the transition state,. Enzymes can lower the activation energy of a chemical reaction in three ways. The activities of enzymes depend on the temperature, ionic conditions, and the ph of the surroundings. The increased rate is the same in. Explain How Enzymes Lower Activation Energy.
From www.slideserve.com
PPT Metabolism PowerPoint Presentation, free download ID1153506 Explain How Enzymes Lower Activation Energy The activation energy (ea) is actually the energy required to form the transition state, so enzymes lower the ea by stabilising the transition state,. This means their shape (as well as the shape of the active site of an enzyme) is determined by the complex. The increased rate is the same in both. Enzymes (and other catalysts) act by reducing. Explain How Enzymes Lower Activation Energy.
From www.slideserve.com
PPT EnZyMeS PowerPoint Presentation, free download ID2065739 Explain How Enzymes Lower Activation Energy Enzymes work by lowering the activation energy needed to start biochemical reactions. The increased rate is the same in both. The activities of enzymes depend on the temperature, ionic conditions, and the ph of the surroundings. This means their shape (as well as the shape of the active site of an enzyme) is determined by the complex. Enzymes (and other. Explain How Enzymes Lower Activation Energy.
From www.albert.io
Enzymes AP® Biology Crash Course Review Albert.io Explain How Enzymes Lower Activation Energy Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. This means their shape (as well as the shape of the active site of an enzyme) is determined by the complex. The way enzymes lower activation energy is by providing an alternative reaction pathway with a lower activation energy. Enzymes work by lowering the. Explain How Enzymes Lower Activation Energy.
From www.pinterest.com
Enzymes Lower Activation Energy A Level Biology Revision Notes A Explain How Enzymes Lower Activation Energy This means their shape (as well as the shape of the active site of an enzyme) is determined by the complex. The activities of enzymes depend on the temperature, ionic conditions, and the ph of the surroundings. Enzymes work by lowering the activation energy needed to start biochemical reactions. Enzymes can lower the activation energy of a chemical reaction in. Explain How Enzymes Lower Activation Energy.
From slideplayer.com
SB1b. Explain how enzymes function as catalysts. ppt download Explain How Enzymes Lower Activation Energy The activation energy (ea) is actually the energy required to form the transition state, so enzymes lower the ea by stabilising the transition state,. The activities of enzymes depend on the temperature, ionic conditions, and the ph of the surroundings. One of the ways the activation energy is lowered is having the. This means their shape (as well as the. Explain How Enzymes Lower Activation Energy.
From btccasting.weebly.com
Enzymes Lower The Activation Energy Of A Reaction btccasting Explain How Enzymes Lower Activation Energy One of the ways the activation energy is lowered is having the. Enzymes work by lowering the activation energy needed to start biochemical reactions. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. Enzymes can lower the activation energy of a chemical reaction in three ways. The way enzymes lower activation energy is. Explain How Enzymes Lower Activation Energy.
From studylib.net
Enzymes Lower Activation Energy Explain How Enzymes Lower Activation Energy This means their shape (as well as the shape of the active site of an enzyme) is determined by the complex. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. The activation energy (ea) is actually the energy required to form the transition state, so enzymes lower the ea by stabilising the transition. Explain How Enzymes Lower Activation Energy.
From brainly.com
12.) Using graph 1, explain how enzymes work. Include the term Explain How Enzymes Lower Activation Energy The way enzymes lower activation energy is by providing an alternative reaction pathway with a lower activation energy. The activation energy (ea) is actually the energy required to form the transition state, so enzymes lower the ea by stabilising the transition state,. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. Enzymes can. Explain How Enzymes Lower Activation Energy.
From zymvol.com
All you need to know about enzymes Zymvol Explain How Enzymes Lower Activation Energy The activation energy (ea) is actually the energy required to form the transition state, so enzymes lower the ea by stabilising the transition state,. One of the ways the activation energy is lowered is having the. This means their shape (as well as the shape of the active site of an enzyme) is determined by the complex. Enzymes (and other. Explain How Enzymes Lower Activation Energy.
From www.genome.gov
Enzyme Explain How Enzymes Lower Activation Energy Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. The activities of enzymes depend on the temperature, ionic conditions, and the ph of the surroundings. The activation energy (ea) is actually the energy required to form the transition state, so enzymes lower the ea by stabilising the transition state,. One of the ways. Explain How Enzymes Lower Activation Energy.
From linfeibioblog.blogspot.com
Linfei's Bio Blog Enzymes Explain How Enzymes Lower Activation Energy Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. This means their shape (as well as the shape of the active site of an enzyme) is determined by the complex. The activities of enzymes depend on the temperature, ionic conditions, and the ph of the surroundings. Enzymes work by lowering the activation energy. Explain How Enzymes Lower Activation Energy.
From brainly.com
12) Using graph 1, explain how enzymes work. Include the term Explain How Enzymes Lower Activation Energy Enzymes can lower the activation energy of a chemical reaction in three ways. The activation energy (ea) is actually the energy required to form the transition state, so enzymes lower the ea by stabilising the transition state,. The activities of enzymes depend on the temperature, ionic conditions, and the ph of the surroundings. Enzymes (and other catalysts) act by reducing. Explain How Enzymes Lower Activation Energy.
From biology4ibdp.weebly.com
2.5 Enzymes BIOLOGY4IBDP Explain How Enzymes Lower Activation Energy Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. The increased rate is the same in both. The activities of enzymes depend on the temperature, ionic conditions, and the ph of the surroundings. Enzymes work by lowering the activation energy needed to start biochemical reactions. One of the ways the activation energy is. Explain How Enzymes Lower Activation Energy.
From msqbio11.blogspot.com
Grade 11 Chapter 4 ENZYMES Explain How Enzymes Lower Activation Energy One of the ways the activation energy is lowered is having the. Enzymes can lower the activation energy of a chemical reaction in three ways. The activation energy (ea) is actually the energy required to form the transition state, so enzymes lower the ea by stabilising the transition state,. Enzymes (and other catalysts) act by reducing the activation energy, thereby. Explain How Enzymes Lower Activation Energy.