Submitted To Qc Review . In medical writing, quality control (qc) means ensuring that a document’s content, style, and. Quality control (qc) review process. Is one faster than the. This document provides an overview of the clinicaltrials.gov. Are they the same thing? It should occur before your project is submitted to your review team for the first time, but you should look at the project timeline and try to identify which day you plan to send your document and source files to your qc specialist—and when they will return their comments to you. I seen people who have had either status after “submitted to qc review”. Consistent with 42 cfr part 11, the national library of medicine (nlm) publicly posts results information within 30 days of submission,. Clinicaltrials.gov results quality control review criteria: The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information.
from template.ourinsurance.web.id
Clinicaltrials.gov results quality control review criteria: Are they the same thing? In medical writing, quality control (qc) means ensuring that a document’s content, style, and. It should occur before your project is submitted to your review team for the first time, but you should look at the project timeline and try to identify which day you plan to send your document and source files to your qc specialist—and when they will return their comments to you. This document provides an overview of the clinicaltrials.gov. Is one faster than the. Consistent with 42 cfr part 11, the national library of medicine (nlm) publicly posts results information within 30 days of submission,. I seen people who have had either status after “submitted to qc review”. Quality control (qc) review process. The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information.
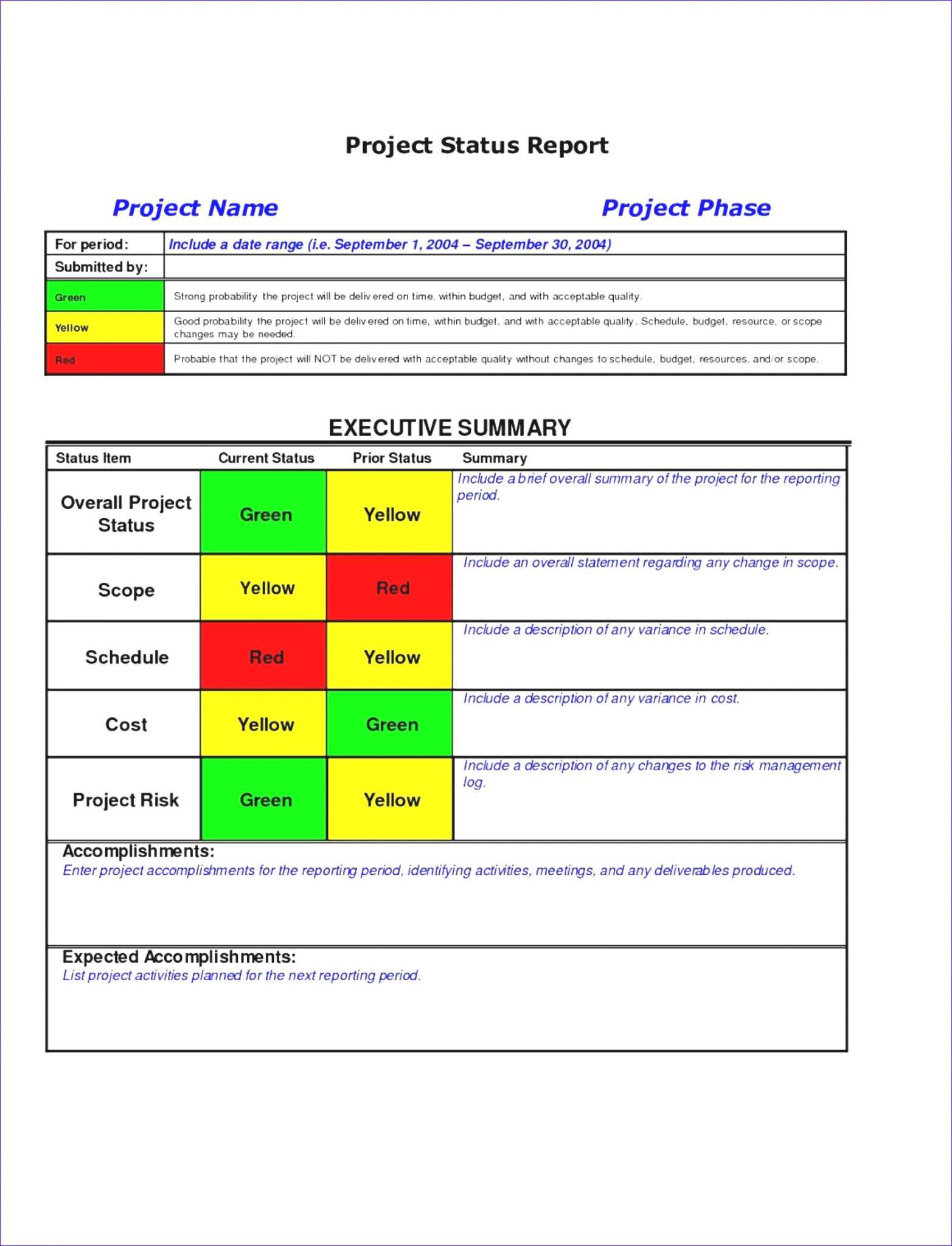
Qa Qc Report Template And Sample With Customisable Format within
Submitted To Qc Review Clinicaltrials.gov results quality control review criteria: It should occur before your project is submitted to your review team for the first time, but you should look at the project timeline and try to identify which day you plan to send your document and source files to your qc specialist—and when they will return their comments to you. This document provides an overview of the clinicaltrials.gov. Is one faster than the. In medical writing, quality control (qc) means ensuring that a document’s content, style, and. Quality control (qc) review process. The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information. Are they the same thing? Consistent with 42 cfr part 11, the national library of medicine (nlm) publicly posts results information within 30 days of submission,. I seen people who have had either status after “submitted to qc review”. Clinicaltrials.gov results quality control review criteria:
From www.slideteam.net
Key Steps Of QA QC Process For Quality Management PPT Presentation Submitted To Qc Review This document provides an overview of the clinicaltrials.gov. It should occur before your project is submitted to your review team for the first time, but you should look at the project timeline and try to identify which day you plan to send your document and source files to your qc specialist—and when they will return their comments to you. Are. Submitted To Qc Review.
From template.ourinsurance.web.id
Qa Qc Report Template And Sample With Customisable Format within Submitted To Qc Review Quality control (qc) review process. Is one faster than the. Consistent with 42 cfr part 11, the national library of medicine (nlm) publicly posts results information within 30 days of submission,. Are they the same thing? Clinicaltrials.gov results quality control review criteria: The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information. In medical writing,. Submitted To Qc Review.
From insight-quality.com
Example Quality Inspection Checklists Insight Quality Services Submitted To Qc Review In medical writing, quality control (qc) means ensuring that a document’s content, style, and. Clinicaltrials.gov results quality control review criteria: Are they the same thing? It should occur before your project is submitted to your review team for the first time, but you should look at the project timeline and try to identify which day you plan to send your. Submitted To Qc Review.
From www.smartlabtools.com
SmartLabTools SLT_QC Review Forms Submitted To Qc Review The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information. Clinicaltrials.gov results quality control review criteria: I seen people who have had either status after “submitted to qc review”. Is one faster than the. It should occur before your project is submitted to your review team for the first time, but you should look at. Submitted To Qc Review.
From www.slideshare.net
QC Review Process Submitted To Qc Review In medical writing, quality control (qc) means ensuring that a document’s content, style, and. Quality control (qc) review process. Consistent with 42 cfr part 11, the national library of medicine (nlm) publicly posts results information within 30 days of submission,. Are they the same thing? The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information.. Submitted To Qc Review.
From www.smartlabtools.com
SmartLabTools SLT_QC Review Forms Submitted To Qc Review Clinicaltrials.gov results quality control review criteria: Are they the same thing? I seen people who have had either status after “submitted to qc review”. Quality control (qc) review process. Is one faster than the. Consistent with 42 cfr part 11, the national library of medicine (nlm) publicly posts results information within 30 days of submission,. This document provides an overview. Submitted To Qc Review.
From www.smartlabtools.com
SmartLabTools SLT_QC Review Forms Submitted To Qc Review The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information. Quality control (qc) review process. It should occur before your project is submitted to your review team for the first time, but you should look at the project timeline and try to identify which day you plan to send your document and source files to. Submitted To Qc Review.
From www.smartlabtools.com
SmartLabTools SLT_QC Review Forms Submitted To Qc Review Consistent with 42 cfr part 11, the national library of medicine (nlm) publicly posts results information within 30 days of submission,. Are they the same thing? Quality control (qc) review process. It should occur before your project is submitted to your review team for the first time, but you should look at the project timeline and try to identify which. Submitted To Qc Review.
From www.slideshare.net
QC Review Process Submitted To Qc Review Clinicaltrials.gov results quality control review criteria: The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information. It should occur before your project is submitted to your review team for the first time, but you should look at the project timeline and try to identify which day you plan to send your document and source files. Submitted To Qc Review.
From www.smartlabtools.com
SmartLabTools SLT_QC Review Forms Submitted To Qc Review Quality control (qc) review process. In medical writing, quality control (qc) means ensuring that a document’s content, style, and. Clinicaltrials.gov results quality control review criteria: The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information. I seen people who have had either status after “submitted to qc review”. It should occur before your project is. Submitted To Qc Review.
From www.slideshare.net
QC Review Process Submitted To Qc Review Quality control (qc) review process. Are they the same thing? In medical writing, quality control (qc) means ensuring that a document’s content, style, and. This document provides an overview of the clinicaltrials.gov. The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information. Consistent with 42 cfr part 11, the national library of medicine (nlm) publicly. Submitted To Qc Review.
From www.slideshare.net
QC Review Process Submitted To Qc Review I seen people who have had either status after “submitted to qc review”. Is one faster than the. Quality control (qc) review process. It should occur before your project is submitted to your review team for the first time, but you should look at the project timeline and try to identify which day you plan to send your document and. Submitted To Qc Review.
From www.slideshare.net
QC Review Process Submitted To Qc Review Quality control (qc) review process. Consistent with 42 cfr part 11, the national library of medicine (nlm) publicly posts results information within 30 days of submission,. I seen people who have had either status after “submitted to qc review”. The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information. Is one faster than the. Clinicaltrials.gov. Submitted To Qc Review.
From journals.sagepub.com
Quality control review implementing a scientifically based quality Submitted To Qc Review The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information. Clinicaltrials.gov results quality control review criteria: Are they the same thing? Quality control (qc) review process. Is one faster than the. In medical writing, quality control (qc) means ensuring that a document’s content, style, and. I seen people who have had either status after “submitted. Submitted To Qc Review.
From sitemate.com
QA QC Report template and sample with customisable format Submitted To Qc Review The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information. This document provides an overview of the clinicaltrials.gov. Consistent with 42 cfr part 11, the national library of medicine (nlm) publicly posts results information within 30 days of submission,. I seen people who have had either status after “submitted to qc review”. Quality control (qc). Submitted To Qc Review.
From www.slideserve.com
PPT Data Management in Pharmacovigilance PowerPoint Presentation Submitted To Qc Review This document provides an overview of the clinicaltrials.gov. The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information. Quality control (qc) review process. Is one faster than the. Are they the same thing? Clinicaltrials.gov results quality control review criteria: Consistent with 42 cfr part 11, the national library of medicine (nlm) publicly posts results information. Submitted To Qc Review.
From www.smartlabtools.com
SmartLabTools SLT_QC Review Forms Submitted To Qc Review In medical writing, quality control (qc) means ensuring that a document’s content, style, and. I seen people who have had either status after “submitted to qc review”. Clinicaltrials.gov results quality control review criteria: Are they the same thing? Quality control (qc) review process. This document provides an overview of the clinicaltrials.gov. It should occur before your project is submitted to. Submitted To Qc Review.
From www.slideteam.net
Top 5 Quality Inspection Report Templates with Samples and Examples Submitted To Qc Review It should occur before your project is submitted to your review team for the first time, but you should look at the project timeline and try to identify which day you plan to send your document and source files to your qc specialist—and when they will return their comments to you. Quality control (qc) review process. Consistent with 42 cfr. Submitted To Qc Review.
From www.slideshare.net
QC Review Process Submitted To Qc Review Consistent with 42 cfr part 11, the national library of medicine (nlm) publicly posts results information within 30 days of submission,. The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information. Is one faster than the. Clinicaltrials.gov results quality control review criteria: Are they the same thing? I seen people who have had either status. Submitted To Qc Review.
From www.smartlabtools.com
SmartLabTools SLT_QC Review Forms Submitted To Qc Review This document provides an overview of the clinicaltrials.gov. The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information. I seen people who have had either status after “submitted to qc review”. It should occur before your project is submitted to your review team for the first time, but you should look at the project timeline. Submitted To Qc Review.
From www.slideshare.net
QC Review Process Submitted To Qc Review Is one faster than the. The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information. Consistent with 42 cfr part 11, the national library of medicine (nlm) publicly posts results information within 30 days of submission,. In medical writing, quality control (qc) means ensuring that a document’s content, style, and. I seen people who have. Submitted To Qc Review.
From jingsourcing.com
QC & QA How to do QC (Methods & Process) Submitted To Qc Review Is one faster than the. Clinicaltrials.gov results quality control review criteria: This document provides an overview of the clinicaltrials.gov. I seen people who have had either status after “submitted to qc review”. Quality control (qc) review process. Consistent with 42 cfr part 11, the national library of medicine (nlm) publicly posts results information within 30 days of submission,. The clinicaltrials.gov. Submitted To Qc Review.
From www.smartlabtools.com
SmartLabTools SLT_QC Review Forms Submitted To Qc Review Are they the same thing? This document provides an overview of the clinicaltrials.gov. In medical writing, quality control (qc) means ensuring that a document’s content, style, and. Is one faster than the. I seen people who have had either status after “submitted to qc review”. Clinicaltrials.gov results quality control review criteria: The clinicaltrials.gov prs team performs a quality control (qc). Submitted To Qc Review.
From www.slideshare.net
QC Review Process Submitted To Qc Review Clinicaltrials.gov results quality control review criteria: Quality control (qc) review process. I seen people who have had either status after “submitted to qc review”. The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information. Consistent with 42 cfr part 11, the national library of medicine (nlm) publicly posts results information within 30 days of submission,.. Submitted To Qc Review.
From sitemate.com
QA QC Report template and sample with customisable format Submitted To Qc Review Is one faster than the. I seen people who have had either status after “submitted to qc review”. This document provides an overview of the clinicaltrials.gov. Quality control (qc) review process. Clinicaltrials.gov results quality control review criteria: The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information. Are they the same thing? Consistent with 42. Submitted To Qc Review.
From www.reddit.com
My status still reads “submitted to QC review” but supervisor states my Submitted To Qc Review The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information. Clinicaltrials.gov results quality control review criteria: This document provides an overview of the clinicaltrials.gov. Consistent with 42 cfr part 11, the national library of medicine (nlm) publicly posts results information within 30 days of submission,. Are they the same thing? Quality control (qc) review process.. Submitted To Qc Review.
From www.slideteam.net
Comparison Matrix Of QA QC And Testing PPT Sample Submitted To Qc Review Is one faster than the. The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information. Consistent with 42 cfr part 11, the national library of medicine (nlm) publicly posts results information within 30 days of submission,. I seen people who have had either status after “submitted to qc review”. Clinicaltrials.gov results quality control review criteria:. Submitted To Qc Review.
From www.smartlabtools.com
SmartLabTools SLT_QC Review Forms Submitted To Qc Review This document provides an overview of the clinicaltrials.gov. I seen people who have had either status after “submitted to qc review”. Clinicaltrials.gov results quality control review criteria: It should occur before your project is submitted to your review team for the first time, but you should look at the project timeline and try to identify which day you plan to. Submitted To Qc Review.
From www.smartlabtools.com
SmartLabTools SLT_QC Review Forms Submitted To Qc Review Quality control (qc) review process. I seen people who have had either status after “submitted to qc review”. In medical writing, quality control (qc) means ensuring that a document’s content, style, and. It should occur before your project is submitted to your review team for the first time, but you should look at the project timeline and try to identify. Submitted To Qc Review.
From mungfali.com
Quality Management Review Template Submitted To Qc Review In medical writing, quality control (qc) means ensuring that a document’s content, style, and. This document provides an overview of the clinicaltrials.gov. Clinicaltrials.gov results quality control review criteria: Is one faster than the. I seen people who have had either status after “submitted to qc review”. The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results. Submitted To Qc Review.
From www.slideshare.net
QC Review Process Submitted To Qc Review Is one faster than the. The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information. In medical writing, quality control (qc) means ensuring that a document’s content, style, and. I seen people who have had either status after “submitted to qc review”. Are they the same thing? Clinicaltrials.gov results quality control review criteria: Quality control. Submitted To Qc Review.
From www.slideshare.net
QC Review Process Submitted To Qc Review The clinicaltrials.gov prs team performs a quality control (qc) review to validate the results information. Is one faster than the. This document provides an overview of the clinicaltrials.gov. Quality control (qc) review process. I seen people who have had either status after “submitted to qc review”. Consistent with 42 cfr part 11, the national library of medicine (nlm) publicly posts. Submitted To Qc Review.
From www.examples.com
Quality Checklist 11+ Examples, Format, Pdf Submitted To Qc Review Is one faster than the. Quality control (qc) review process. I seen people who have had either status after “submitted to qc review”. It should occur before your project is submitted to your review team for the first time, but you should look at the project timeline and try to identify which day you plan to send your document and. Submitted To Qc Review.
From www.slideshare.net
QC Review Process Submitted To Qc Review This document provides an overview of the clinicaltrials.gov. Are they the same thing? Clinicaltrials.gov results quality control review criteria: It should occur before your project is submitted to your review team for the first time, but you should look at the project timeline and try to identify which day you plan to send your document and source files to your. Submitted To Qc Review.
From www.slideshare.net
QC Review Process Submitted To Qc Review This document provides an overview of the clinicaltrials.gov. I seen people who have had either status after “submitted to qc review”. It should occur before your project is submitted to your review team for the first time, but you should look at the project timeline and try to identify which day you plan to send your document and source files. Submitted To Qc Review.