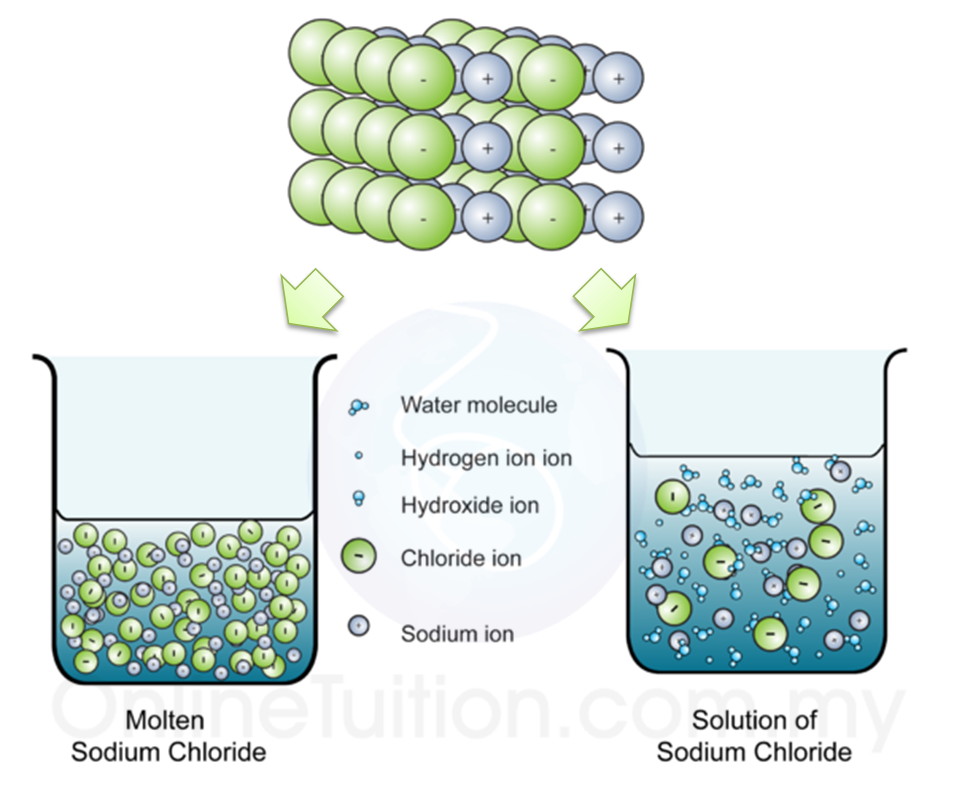
Molten Electrolyte Examples . This demonstration does not need too much preparation and the apparatus involved is very straightforward. Let’s examine two examples to predict the processes and products of electrolysis in molten electrolytes. The electrolysis of molten ionic compounds. New substances form when a. Examples of electrolysing molten compounds. A binary ionic compound is one consisting of just two elements joined together by ionic bonding. An electrolyte is a compound which undergoes electrolysis. Introduce your students to the study of electrolysis by demonstrating how conduction is only possible where lead (ii) bromide is molten, and that metallic lead and bromine are the products of the molten electrolyte. The products of electrolysis can be predicted for a. Electrolysis involves using electricity to break down electrolytes to form elements. This page looks in detail at the electrolysis of molten ionic compounds such as lead (ii) bromide, zinc. The reason that an electrolyte has to be molten or in solution is that the current is carried through the electrolyte by the.
from proquestyamaha.web.fc2.com
Let’s examine two examples to predict the processes and products of electrolysis in molten electrolytes. This page looks in detail at the electrolysis of molten ionic compounds such as lead (ii) bromide, zinc. This demonstration does not need too much preparation and the apparatus involved is very straightforward. The products of electrolysis can be predicted for a. The electrolysis of molten ionic compounds. Introduce your students to the study of electrolysis by demonstrating how conduction is only possible where lead (ii) bromide is molten, and that metallic lead and bromine are the products of the molten electrolyte. The reason that an electrolyte has to be molten or in solution is that the current is carried through the electrolyte by the. A binary ionic compound is one consisting of just two elements joined together by ionic bonding. Electrolysis involves using electricity to break down electrolytes to form elements. New substances form when a.
Is sodium chloride an electrolyte?
Molten Electrolyte Examples New substances form when a. A binary ionic compound is one consisting of just two elements joined together by ionic bonding. The electrolysis of molten ionic compounds. Let’s examine two examples to predict the processes and products of electrolysis in molten electrolytes. New substances form when a. Electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a. Examples of electrolysing molten compounds. This demonstration does not need too much preparation and the apparatus involved is very straightforward. This page looks in detail at the electrolysis of molten ionic compounds such as lead (ii) bromide, zinc. An electrolyte is a compound which undergoes electrolysis. Introduce your students to the study of electrolysis by demonstrating how conduction is only possible where lead (ii) bromide is molten, and that metallic lead and bromine are the products of the molten electrolyte. The reason that an electrolyte has to be molten or in solution is that the current is carried through the electrolyte by the.
From www.pinterest.com
What is Electrolysis and Electrolyte with examples. Teaching Molten Electrolyte Examples An electrolyte is a compound which undergoes electrolysis. This page looks in detail at the electrolysis of molten ionic compounds such as lead (ii) bromide, zinc. Let’s examine two examples to predict the processes and products of electrolysis in molten electrolytes. Electrolysis involves using electricity to break down electrolytes to form elements. The electrolysis of molten ionic compounds. A binary. Molten Electrolyte Examples.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Molten Electrolyte Examples Examples of electrolysing molten compounds. Introduce your students to the study of electrolysis by demonstrating how conduction is only possible where lead (ii) bromide is molten, and that metallic lead and bromine are the products of the molten electrolyte. The electrolysis of molten ionic compounds. An electrolyte is a compound which undergoes electrolysis. This page looks in detail at the. Molten Electrolyte Examples.
From www.pinnaxis.com
Molten Electrolyte Examples Online Factory Molten Electrolyte Examples The reason that an electrolyte has to be molten or in solution is that the current is carried through the electrolyte by the. This page looks in detail at the electrolysis of molten ionic compounds such as lead (ii) bromide, zinc. Examples of electrolysing molten compounds. New substances form when a. Let’s examine two examples to predict the processes and. Molten Electrolyte Examples.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Molten Electrolyte Examples A binary ionic compound is one consisting of just two elements joined together by ionic bonding. Examples of electrolysing molten compounds. An electrolyte is a compound which undergoes electrolysis. The products of electrolysis can be predicted for a. This page looks in detail at the electrolysis of molten ionic compounds such as lead (ii) bromide, zinc. The electrolysis of molten. Molten Electrolyte Examples.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID5813936 Molten Electrolyte Examples A binary ionic compound is one consisting of just two elements joined together by ionic bonding. An electrolyte is a compound which undergoes electrolysis. Introduce your students to the study of electrolysis by demonstrating how conduction is only possible where lead (ii) bromide is molten, and that metallic lead and bromine are the products of the molten electrolyte. This demonstration. Molten Electrolyte Examples.
From mantavya.com
What Is Electroplating & How does it work 2021 Guide Mantavya Molten Electrolyte Examples The products of electrolysis can be predicted for a. A binary ionic compound is one consisting of just two elements joined together by ionic bonding. New substances form when a. This demonstration does not need too much preparation and the apparatus involved is very straightforward. Examples of electrolysing molten compounds. An electrolyte is a compound which undergoes electrolysis. The reason. Molten Electrolyte Examples.
From studylib.net
Molten electrolyte Molten Electrolyte Examples Electrolysis involves using electricity to break down electrolytes to form elements. This page looks in detail at the electrolysis of molten ionic compounds such as lead (ii) bromide, zinc. Examples of electrolysing molten compounds. The electrolysis of molten ionic compounds. An electrolyte is a compound which undergoes electrolysis. New substances form when a. The products of electrolysis can be predicted. Molten Electrolyte Examples.
From slidetodoc.com
Introduction Molten electrolyte Aqueous electrolyte Summary We have Molten Electrolyte Examples Let’s examine two examples to predict the processes and products of electrolysis in molten electrolytes. This page looks in detail at the electrolysis of molten ionic compounds such as lead (ii) bromide, zinc. An electrolyte is a compound which undergoes electrolysis. Introduce your students to the study of electrolysis by demonstrating how conduction is only possible where lead (ii) bromide. Molten Electrolyte Examples.
From www.youtube.com
Identifying Strong Electrolytes, Weak Electrolytes, and Nonelectrolytes Molten Electrolyte Examples Examples of electrolysing molten compounds. This page looks in detail at the electrolysis of molten ionic compounds such as lead (ii) bromide, zinc. An electrolyte is a compound which undergoes electrolysis. Electrolysis involves using electricity to break down electrolytes to form elements. This demonstration does not need too much preparation and the apparatus involved is very straightforward. Introduce your students. Molten Electrolyte Examples.
From slidetodoc.com
Introduction Molten electrolyte Aqueous electrolyte Summary We have Molten Electrolyte Examples Let’s examine two examples to predict the processes and products of electrolysis in molten electrolytes. This page looks in detail at the electrolysis of molten ionic compounds such as lead (ii) bromide, zinc. An electrolyte is a compound which undergoes electrolysis. This demonstration does not need too much preparation and the apparatus involved is very straightforward. A binary ionic compound. Molten Electrolyte Examples.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Molten Electrolyte Examples New substances form when a. The reason that an electrolyte has to be molten or in solution is that the current is carried through the electrolyte by the. Electrolysis involves using electricity to break down electrolytes to form elements. Let’s examine two examples to predict the processes and products of electrolysis in molten electrolytes. Introduce your students to the study. Molten Electrolyte Examples.
From www.slideserve.com
PPT Electrolysis of Salt Water PowerPoint Presentation, free download Molten Electrolyte Examples Electrolysis involves using electricity to break down electrolytes to form elements. A binary ionic compound is one consisting of just two elements joined together by ionic bonding. Introduce your students to the study of electrolysis by demonstrating how conduction is only possible where lead (ii) bromide is molten, and that metallic lead and bromine are the products of the molten. Molten Electrolyte Examples.
From www.slideserve.com
PPT Electrolysis L.O. I know and can use the terms electrolyte Molten Electrolyte Examples The reason that an electrolyte has to be molten or in solution is that the current is carried through the electrolyte by the. Electrolysis involves using electricity to break down electrolytes to form elements. An electrolyte is a compound which undergoes electrolysis. This demonstration does not need too much preparation and the apparatus involved is very straightforward. The products of. Molten Electrolyte Examples.
From www.youtube.com
Electrolysis of Molten NaCl (in Urdu/Hindi) Oxidation and Reduction Molten Electrolyte Examples The products of electrolysis can be predicted for a. Let’s examine two examples to predict the processes and products of electrolysis in molten electrolytes. The electrolysis of molten ionic compounds. The reason that an electrolyte has to be molten or in solution is that the current is carried through the electrolyte by the. This page looks in detail at the. Molten Electrolyte Examples.
From www.elevise.co.uk
C4 L) Electrolysis Part 2 AQA Chemistry Elevise Molten Electrolyte Examples The products of electrolysis can be predicted for a. Electrolysis involves using electricity to break down electrolytes to form elements. An electrolyte is a compound which undergoes electrolysis. Let’s examine two examples to predict the processes and products of electrolysis in molten electrolytes. A binary ionic compound is one consisting of just two elements joined together by ionic bonding. New. Molten Electrolyte Examples.
From ar.inspiredpencil.com
Electrolyte Examples Chemistry Molten Electrolyte Examples A binary ionic compound is one consisting of just two elements joined together by ionic bonding. Electrolysis involves using electricity to break down electrolytes to form elements. New substances form when a. This page looks in detail at the electrolysis of molten ionic compounds such as lead (ii) bromide, zinc. The electrolysis of molten ionic compounds. Introduce your students to. Molten Electrolyte Examples.
From www.vrogue.co
Weak Electrolyte Definition And Examples vrogue.co Molten Electrolyte Examples This page looks in detail at the electrolysis of molten ionic compounds such as lead (ii) bromide, zinc. New substances form when a. Electrolysis involves using electricity to break down electrolytes to form elements. The electrolysis of molten ionic compounds. An electrolyte is a compound which undergoes electrolysis. Introduce your students to the study of electrolysis by demonstrating how conduction. Molten Electrolyte Examples.
From teraznews.com
Which of the Following Compounds is a Weak Electrolyte Molten Electrolyte Examples An electrolyte is a compound which undergoes electrolysis. Examples of electrolysing molten compounds. Electrolysis involves using electricity to break down electrolytes to form elements. Introduce your students to the study of electrolysis by demonstrating how conduction is only possible where lead (ii) bromide is molten, and that metallic lead and bromine are the products of the molten electrolyte. The reason. Molten Electrolyte Examples.
From ar.inspiredpencil.com
Electrolyte Examples Chemistry Molten Electrolyte Examples A binary ionic compound is one consisting of just two elements joined together by ionic bonding. Examples of electrolysing molten compounds. The reason that an electrolyte has to be molten or in solution is that the current is carried through the electrolyte by the. This page looks in detail at the electrolysis of molten ionic compounds such as lead (ii). Molten Electrolyte Examples.
From vectormine.com
Anode and cathode scientific physics education diagram, vector Molten Electrolyte Examples A binary ionic compound is one consisting of just two elements joined together by ionic bonding. The electrolysis of molten ionic compounds. New substances form when a. Introduce your students to the study of electrolysis by demonstrating how conduction is only possible where lead (ii) bromide is molten, and that metallic lead and bromine are the products of the molten. Molten Electrolyte Examples.
From www.myxxgirl.com
Electrolyte Definition Examples Study Com My XXX Hot Girl Molten Electrolyte Examples Examples of electrolysing molten compounds. The products of electrolysis can be predicted for a. This page looks in detail at the electrolysis of molten ionic compounds such as lead (ii) bromide, zinc. The reason that an electrolyte has to be molten or in solution is that the current is carried through the electrolyte by the. A binary ionic compound is. Molten Electrolyte Examples.
From www.shutterstock.com
413 Electrolytic solution Gambar, Foto Stok & Vektor Shutterstock Molten Electrolyte Examples An electrolyte is a compound which undergoes electrolysis. The reason that an electrolyte has to be molten or in solution is that the current is carried through the electrolyte by the. A binary ionic compound is one consisting of just two elements joined together by ionic bonding. Let’s examine two examples to predict the processes and products of electrolysis in. Molten Electrolyte Examples.
From slideplayer.com
The ionic compound is called an electrolyte. ppt download Molten Electrolyte Examples The reason that an electrolyte has to be molten or in solution is that the current is carried through the electrolyte by the. Examples of electrolysing molten compounds. Introduce your students to the study of electrolysis by demonstrating how conduction is only possible where lead (ii) bromide is molten, and that metallic lead and bromine are the products of the. Molten Electrolyte Examples.
From ar.inspiredpencil.com
Electrolyte Examples Chemistry Molten Electrolyte Examples The reason that an electrolyte has to be molten or in solution is that the current is carried through the electrolyte by the. This demonstration does not need too much preparation and the apparatus involved is very straightforward. Let’s examine two examples to predict the processes and products of electrolysis in molten electrolytes. A binary ionic compound is one consisting. Molten Electrolyte Examples.
From www.aakash.ac.in
Electrolytes and Nonelectrolytes Types, Differences & Examples AESL Molten Electrolyte Examples Let’s examine two examples to predict the processes and products of electrolysis in molten electrolytes. A binary ionic compound is one consisting of just two elements joined together by ionic bonding. This demonstration does not need too much preparation and the apparatus involved is very straightforward. This page looks in detail at the electrolysis of molten ionic compounds such as. Molten Electrolyte Examples.
From webmis.highland.cc.il.us
Electrolysis Molten Electrolyte Examples Introduce your students to the study of electrolysis by demonstrating how conduction is only possible where lead (ii) bromide is molten, and that metallic lead and bromine are the products of the molten electrolyte. A binary ionic compound is one consisting of just two elements joined together by ionic bonding. The reason that an electrolyte has to be molten or. Molten Electrolyte Examples.
From www.youtube.com
GCSE Chemistry Electrolysis Part 1 Basics and Molten Compounds 40 Molten Electrolyte Examples The products of electrolysis can be predicted for a. A binary ionic compound is one consisting of just two elements joined together by ionic bonding. Examples of electrolysing molten compounds. This page looks in detail at the electrolysis of molten ionic compounds such as lead (ii) bromide, zinc. Electrolysis involves using electricity to break down electrolytes to form elements. Introduce. Molten Electrolyte Examples.
From www.youtube.com
Chp 5 ectrolysis of Molten electrolyte Part 2 YouTube Molten Electrolyte Examples This demonstration does not need too much preparation and the apparatus involved is very straightforward. Let’s examine two examples to predict the processes and products of electrolysis in molten electrolytes. A binary ionic compound is one consisting of just two elements joined together by ionic bonding. Introduce your students to the study of electrolysis by demonstrating how conduction is only. Molten Electrolyte Examples.
From www.slideshare.net
6.1 meaning of electrolyte Molten Electrolyte Examples Let’s examine two examples to predict the processes and products of electrolysis in molten electrolytes. The products of electrolysis can be predicted for a. This page looks in detail at the electrolysis of molten ionic compounds such as lead (ii) bromide, zinc. New substances form when a. An electrolyte is a compound which undergoes electrolysis. Electrolysis involves using electricity to. Molten Electrolyte Examples.
From brainly.in
Modrate electrolyte examples Brainly.in Molten Electrolyte Examples New substances form when a. Introduce your students to the study of electrolysis by demonstrating how conduction is only possible where lead (ii) bromide is molten, and that metallic lead and bromine are the products of the molten electrolyte. Electrolysis involves using electricity to break down electrolytes to form elements. This page looks in detail at the electrolysis of molten. Molten Electrolyte Examples.
From courses.lumenlearning.com
Electrolysis Chemistry Molten Electrolyte Examples A binary ionic compound is one consisting of just two elements joined together by ionic bonding. This demonstration does not need too much preparation and the apparatus involved is very straightforward. Introduce your students to the study of electrolysis by demonstrating how conduction is only possible where lead (ii) bromide is molten, and that metallic lead and bromine are the. Molten Electrolyte Examples.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Molten Electrolyte Examples The electrolysis of molten ionic compounds. The reason that an electrolyte has to be molten or in solution is that the current is carried through the electrolyte by the. This demonstration does not need too much preparation and the apparatus involved is very straightforward. A binary ionic compound is one consisting of just two elements joined together by ionic bonding.. Molten Electrolyte Examples.
From en.ppt-online.org
Aqueous Solutions of Electrolytes online presentation Molten Electrolyte Examples This demonstration does not need too much preparation and the apparatus involved is very straightforward. A binary ionic compound is one consisting of just two elements joined together by ionic bonding. The reason that an electrolyte has to be molten or in solution is that the current is carried through the electrolyte by the. An electrolyte is a compound which. Molten Electrolyte Examples.
From slideplayer.com
Unit 9 Water, Solutions & Reaction Rates ppt download Molten Electrolyte Examples Examples of electrolysing molten compounds. Electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a. The electrolysis of molten ionic compounds. Introduce your students to the study of electrolysis by demonstrating how conduction is only possible where lead (ii) bromide is molten, and that metallic lead and bromine are the. Molten Electrolyte Examples.
From proquestyamaha.web.fc2.com
Is sodium chloride an electrolyte? Molten Electrolyte Examples This page looks in detail at the electrolysis of molten ionic compounds such as lead (ii) bromide, zinc. Let’s examine two examples to predict the processes and products of electrolysis in molten electrolytes. The electrolysis of molten ionic compounds. New substances form when a. The reason that an electrolyte has to be molten or in solution is that the current. Molten Electrolyte Examples.