What Is Heat Capacity Equation . Q = mcδt, where q is the symbol for heat transfer, m is the mass of. The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of. The heat capacity (c) can be calculated by multiplying the specific heat with the mass. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of. Temperature is a measure of the average kinetic energy of the molecules. Different materials require different amounts of. The quantitative relationship between heat transfer and temperature change contains all three factors: Therefore, \ [ c = mc \\ \text {or, } c = \frac {q} {\delta t} \] units. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a.
from www.youtube.com
The heat capacity (c) can be calculated by multiplying the specific heat with the mass. The quantitative relationship between heat transfer and temperature change contains all three factors: Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Q = mcδt, where q is the symbol for heat transfer, m is the mass of. Temperature is a measure of the average kinetic energy of the molecules. Different materials require different amounts of. The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of. Therefore, \ [ c = mc \\ \text {or, } c = \frac {q} {\delta t} \] units.
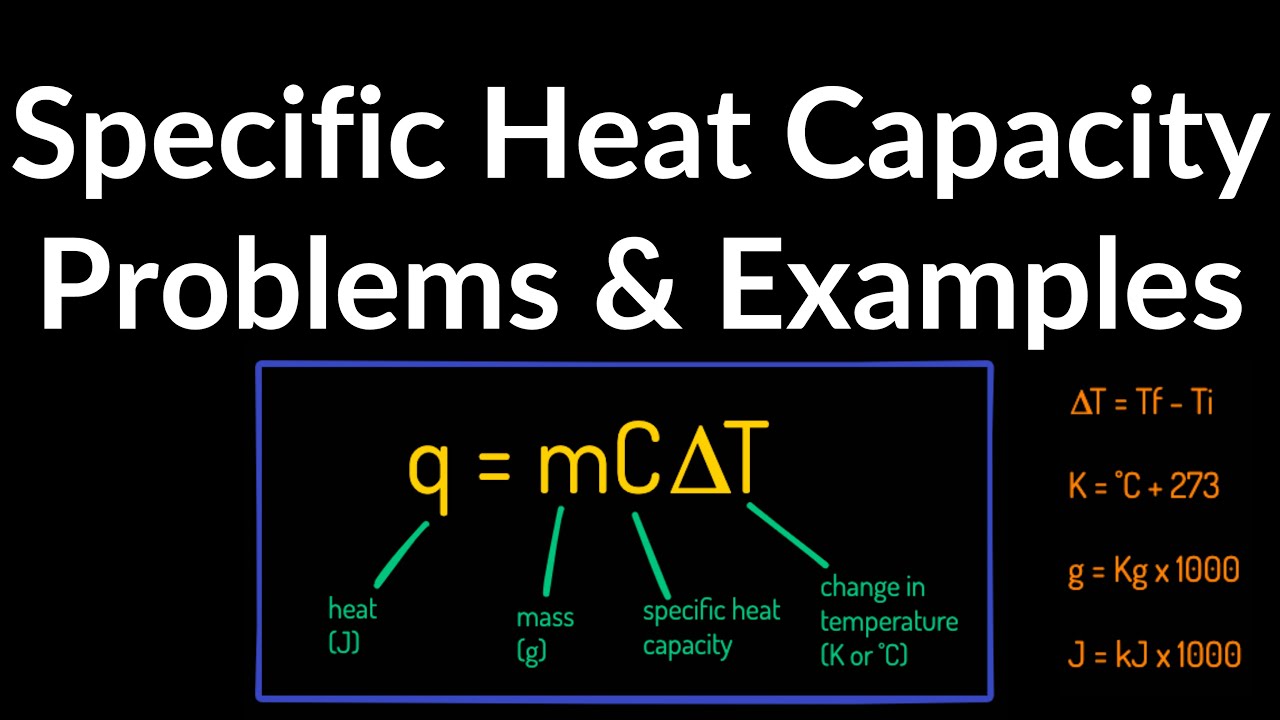
Specific Heat Capacity (q=mC∆T) Examples, Practice Problems, Initial
What Is Heat Capacity Equation Different materials require different amounts of. Different materials require different amounts of. The heat capacity (c) can be calculated by multiplying the specific heat with the mass. The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of. Q = mcδt, where q is the symbol for heat transfer, m is the mass of. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. The quantitative relationship between heat transfer and temperature change contains all three factors: Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of. Temperature is a measure of the average kinetic energy of the molecules. Therefore, \ [ c = mc \\ \text {or, } c = \frac {q} {\delta t} \] units.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity What Is Heat Capacity Equation Q = mcδt, where q is the symbol for heat transfer, m is the mass of. The heat capacity (c) can be calculated by multiplying the specific heat with the mass. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of. Temperature is a measure of. What Is Heat Capacity Equation.
From www.youtube.com
Thermodynamics (Physics) Lesson 2 Heat Transfer and Specific Heat.avi What Is Heat Capacity Equation Different materials require different amounts of. The quantitative relationship between heat transfer and temperature change contains all three factors: Q = mcδt, where q is the symbol for heat transfer, m is the mass of. Temperature is a measure of the average kinetic energy of the molecules. The specific heat capacity of a substance is the amount of thermal energy. What Is Heat Capacity Equation.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube What Is Heat Capacity Equation Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of. Therefore, \ [ c = mc \\ \text {or, } c = \frac {q} {\delta t} \] units. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. What Is Heat Capacity Equation.
From www.nagwa.com
Question Video Rearranging an Equation to Set Specific Heat Capacity What Is Heat Capacity Equation The quantitative relationship between heat transfer and temperature change contains all three factors: Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of. The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of. Different. What Is Heat Capacity Equation.
From www.slideserve.com
PPT Specific Heat PowerPoint Presentation, free download ID3721637 What Is Heat Capacity Equation The quantitative relationship between heat transfer and temperature change contains all three factors: The heat capacity (c) can be calculated by multiplying the specific heat with the mass. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of. Temperature is a measure of the average kinetic. What Is Heat Capacity Equation.
From studymind.co.uk
Heat Capacity Study Mind What Is Heat Capacity Equation Therefore, \ [ c = mc \\ \text {or, } c = \frac {q} {\delta t} \] units. Temperature is a measure of the average kinetic energy of the molecules. The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of. Heat capacity (usually denoted by a capital c,. What Is Heat Capacity Equation.
From www.tes.com
GCSE Physics Specific Heat Capacity Teaching Resources What Is Heat Capacity Equation The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. The heat capacity (c) can be calculated by multiplying the specific heat with the mass.. What Is Heat Capacity Equation.
From www.youtube.com
Specific Heat Capacity (q=mC∆T) Examples, Practice Problems, Initial What Is Heat Capacity Equation Q = mcδt, where q is the symbol for heat transfer, m is the mass of. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of. The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1. What Is Heat Capacity Equation.
From scienceinfo.com
Specific Heat Capacity Definition, Unit, Formula What Is Heat Capacity Equation The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of. The heat capacity of a substance describes how its temperature changes as it absorbs. What Is Heat Capacity Equation.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types What Is Heat Capacity Equation Different materials require different amounts of. Temperature is a measure of the average kinetic energy of the molecules. Q = mcδt, where q is the symbol for heat transfer, m is the mass of. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of. The specific. What Is Heat Capacity Equation.
From www.slideserve.com
PPT ENERGY PowerPoint Presentation, free download ID3232966 What Is Heat Capacity Equation The quantitative relationship between heat transfer and temperature change contains all three factors: The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Q = mcδt, where q is the symbol for heat transfer, m is the mass of. Temperature is a measure of the average kinetic. What Is Heat Capacity Equation.
From studylib.net
Heat Equation What Is Heat Capacity Equation Temperature is a measure of the average kinetic energy of the molecules. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Therefore, \ [ c = mc \\ \text {or, } c = \frac {q} {\delta t} \] units. Q = mcδt, where q is the. What Is Heat Capacity Equation.
From www.youtube.com
Heat Transfer L14 p2 Heat Equation Transient Solution YouTube What Is Heat Capacity Equation The heat capacity (c) can be calculated by multiplying the specific heat with the mass. Different materials require different amounts of. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of. Temperature is a measure of the average kinetic energy of the molecules. The heat capacity. What Is Heat Capacity Equation.
From ar.inspiredpencil.com
Heat Capacity Equation What Is Heat Capacity Equation Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of. Therefore, \ [ c = mc \\ \text {or, } c = \frac {q} {\delta t} \] units. Different materials require different amounts of. The quantitative relationship between heat transfer and temperature change contains all three. What Is Heat Capacity Equation.
From www.toppr.com
Specific Heat Formula Definition, Equations, Examples What Is Heat Capacity Equation Different materials require different amounts of. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of. The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of. The heat capacity (c) can be calculated by. What Is Heat Capacity Equation.
From www.sciencefacts.net
Specific Heat and Heat Capacity Definition, Formula, Values, and Problems What Is Heat Capacity Equation The heat capacity (c) can be calculated by multiplying the specific heat with the mass. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the. What Is Heat Capacity Equation.
From brainly.com
How much heat is released upon converting one mole of steam (18.0 g What Is Heat Capacity Equation Temperature is a measure of the average kinetic energy of the molecules. The heat capacity (c) can be calculated by multiplying the specific heat with the mass. The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of. Different materials require different amounts of. The quantitative relationship between heat. What Is Heat Capacity Equation.
From www.slideserve.com
PPT HEAT EQUATION (in Table T) PowerPoint Presentation, free download What Is Heat Capacity Equation Q = mcδt, where q is the symbol for heat transfer, m is the mass of. The heat capacity (c) can be calculated by multiplying the specific heat with the mass. The quantitative relationship between heat transfer and temperature change contains all three factors: Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the. What Is Heat Capacity Equation.
From www.youtube.com
Specific Heat and Heat Capacity YouTube What Is Heat Capacity Equation Therefore, \ [ c = mc \\ \text {or, } c = \frac {q} {\delta t} \] units. The heat capacity (c) can be calculated by multiplying the specific heat with the mass. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. The quantitative relationship between. What Is Heat Capacity Equation.
From www.markedbyteachers.com
Specific Heat Capacity International Baccalaureate Physics Marked What Is Heat Capacity Equation Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of. Temperature is a measure of the average kinetic energy of the molecules. Therefore, \ [ c = mc \\ \text {or, } c = \frac {q} {\delta t} \] units. The heat capacity of a substance. What Is Heat Capacity Equation.
From www.slideserve.com
PPT Specific Heat PowerPoint Presentation, free download ID3721637 What Is Heat Capacity Equation Different materials require different amounts of. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Therefore, \ [ c = mc \\ \text {or, } c = \frac {q} {\delta t} \] units. The heat capacity (c) can be calculated by multiplying the specific heat with. What Is Heat Capacity Equation.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to What Is Heat Capacity Equation The quantitative relationship between heat transfer and temperature change contains all three factors: Therefore, \ [ c = mc \\ \text {or, } c = \frac {q} {\delta t} \] units. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Different materials require different amounts of.. What Is Heat Capacity Equation.
From www.youtube.com
GCSE Physics All Exam Boards Thermal Physics Specific Heat What Is Heat Capacity Equation Temperature is a measure of the average kinetic energy of the molecules. Different materials require different amounts of. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. What Is Heat Capacity Equation.
From www.slideserve.com
PPT Chapter 17 Thermochemistry PowerPoint Presentation, free What Is Heat Capacity Equation The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Temperature is a measure of the average kinetic energy of the molecules. Therefore, \ [ c = mc \\ \text {or, } c = \frac {q} {\delta t} \] units. The quantitative relationship between heat transfer and. What Is Heat Capacity Equation.
From www.youtube.com
Heat Transfer L12 p1 Finite Difference Heat Equation YouTube What Is Heat Capacity Equation The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Q = mcδt, where q is the symbol for heat transfer, m is the mass of. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes. What Is Heat Capacity Equation.
From www.slideserve.com
PPT Heat capacity and Specific Heat PowerPoint Presentation, free What Is Heat Capacity Equation The heat capacity (c) can be calculated by multiplying the specific heat with the mass. Q = mcδt, where q is the symbol for heat transfer, m is the mass of. The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of. The quantitative relationship between heat transfer and. What Is Heat Capacity Equation.
From www.slideserve.com
PPT Learning Rearranging equation for Specific Heat What Is Heat Capacity Equation Therefore, \ [ c = mc \\ \text {or, } c = \frac {q} {\delta t} \] units. The heat capacity (c) can be calculated by multiplying the specific heat with the mass. Temperature is a measure of the average kinetic energy of the molecules. Q = mcδt, where q is the symbol for heat transfer, m is the mass. What Is Heat Capacity Equation.
From www.youtube.com
Specific heat capacity YouTube What Is Heat Capacity Equation The quantitative relationship between heat transfer and temperature change contains all three factors: Temperature is a measure of the average kinetic energy of the molecules. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of. The heat capacity (c) can be calculated by multiplying the specific. What Is Heat Capacity Equation.
From www.grc.nasa.gov
Specific Heats What Is Heat Capacity Equation Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of. Therefore, \ [ c = mc \\ \text {or, } c = \frac {q} {\delta t} \] units. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. What Is Heat Capacity Equation.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is Heat Capacity Equation The heat capacity (c) can be calculated by multiplying the specific heat with the mass. The quantitative relationship between heat transfer and temperature change contains all three factors: The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Therefore, \ [ c = mc \\ \text {or,. What Is Heat Capacity Equation.
From ar.inspiredpencil.com
Heat Capacity Equation What Is Heat Capacity Equation The quantitative relationship between heat transfer and temperature change contains all three factors: Temperature is a measure of the average kinetic energy of the molecules. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. The specific heat capacity of a substance is the amount of thermal. What Is Heat Capacity Equation.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is Heat Capacity Equation Q = mcδt, where q is the symbol for heat transfer, m is the mass of. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of. Temperature is a measure of the average kinetic energy of the molecules. The heat capacity of a substance describes how. What Is Heat Capacity Equation.
From www.slideshare.net
Heat Capacity What Is Heat Capacity Equation The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Temperature is a measure of the average kinetic energy of the molecules. The heat capacity (c) can be calculated by multiplying the specific heat with the mass. Therefore, \ [ c = mc \\ \text {or, }. What Is Heat Capacity Equation.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii What Is Heat Capacity Equation Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of. Therefore, \ [ c = mc \\ \text {or, } c = \frac {q} {\delta t} \] units. Different materials require different amounts of. Temperature is a measure of the average kinetic energy of the molecules.. What Is Heat Capacity Equation.
From www.numerade.com
SOLVED The heat capacity at constant pressure (Cp) is the partial What Is Heat Capacity Equation Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of. The heat capacity (c) can be calculated by multiplying the specific heat with the mass. Temperature is a measure of the average kinetic energy of the molecules. The heat capacity of a substance describes how its. What Is Heat Capacity Equation.