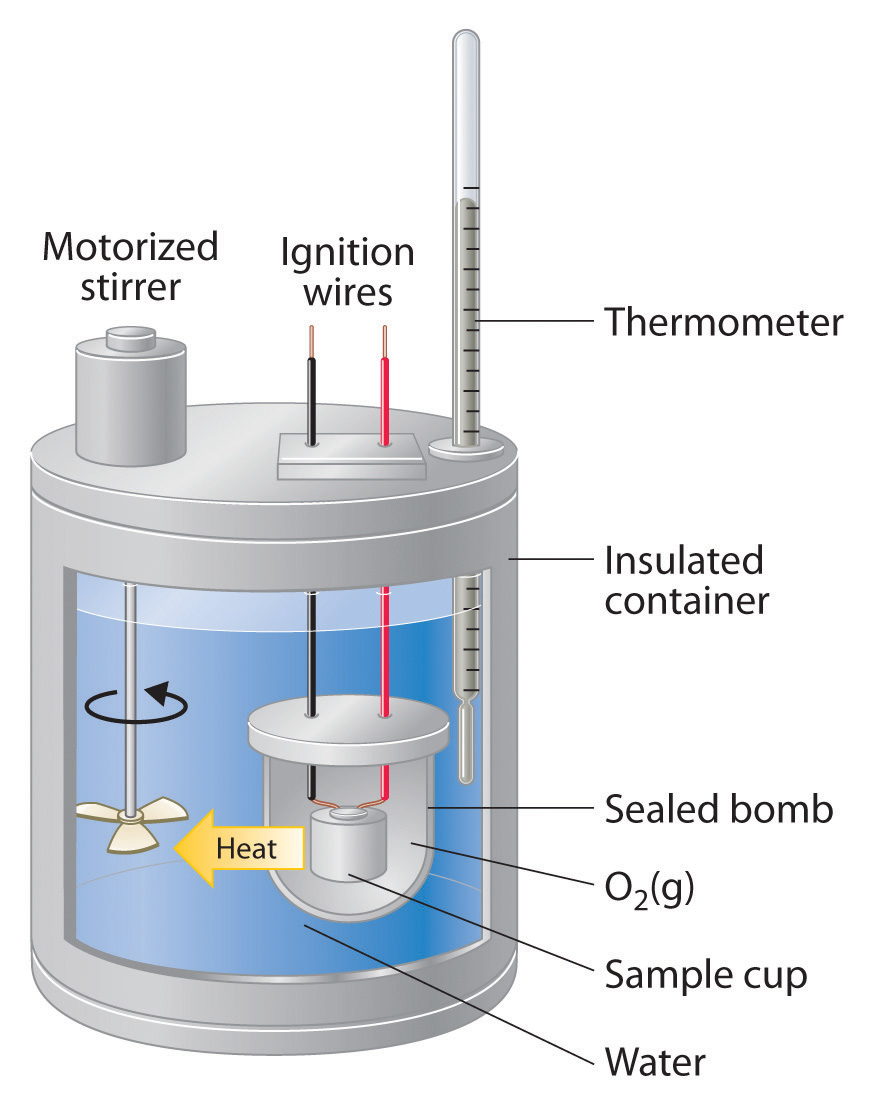
Calibration Of A Bomb Calorimeter Occurs . constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. bomb calorimetry is used to determine the enthalpy of combustion, d combh, for hydrocarbons: bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb). So, for a constant volume, δe = q, and therefore, the bomb calorimeter. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. w = p δ v and δ v is zero when the volume is constant.
from chem.libretexts.org
bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb). w = p δ v and δ v is zero when the volume is constant. bomb calorimetry is used to determine the enthalpy of combustion, d combh, for hydrocarbons: bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and.
6.5 Constant Volume Calorimetry Measuring ΔU for Chemical Reactions
Calibration Of A Bomb Calorimeter Occurs a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb). w = p δ v and δ v is zero when the volume is constant. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimetry is used to determine the enthalpy of combustion, d combh, for hydrocarbons: a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb). bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. So, for a constant volume, δe = q, and therefore, the bomb calorimeter.
From www.youtube.com
Calibration procedure of Bomb calorimeter Why is Benzoic acid used in Calibration Of A Bomb Calorimeter Occurs bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. w = p δ v and δ v is zero when the volume is constant. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. bomb calorimeters require. Calibration Of A Bomb Calorimeter Occurs.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Calibration Of A Bomb Calorimeter Occurs bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb). bomb calorimeters. Calibration Of A Bomb Calorimeter Occurs.
From dxopjumlc.blob.core.windows.net
Calorimeter Calibration Calculator at Jeffery Williams blog Calibration Of A Bomb Calorimeter Occurs bomb calorimetry is used to determine the enthalpy of combustion, d combh, for hydrocarbons: w = p δ v and δ v is zero when the volume is constant. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimeters require calibration to determine the heat capacity of the. Calibration Of A Bomb Calorimeter Occurs.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calibration Of A Bomb Calorimeter Occurs bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb). constant volume. Calibration Of A Bomb Calorimeter Occurs.
From gamma.app
Bomb Calorimeter A Comprehensive Guide Calibration Of A Bomb Calorimeter Occurs a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb). w = p δ v and δ v is zero when the volume is constant. bomb calorimetry is used to determine the enthalpy of combustion, d combh, for hydrocarbons: bomb. Calibration Of A Bomb Calorimeter Occurs.
From www.gauthmath.com
Solved 6. TO CALIBRATE A BOMB CALORIMETER, THE COMBUSTION OF Calibration Of A Bomb Calorimeter Occurs So, for a constant volume, δe = q, and therefore, the bomb calorimeter. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb). bomb calorimeters. Calibration Of A Bomb Calorimeter Occurs.
From www.indiamart.com
Bomb Calorimeter with Automatic Calculation, For Coal And Briquette Calibration Of A Bomb Calorimeter Occurs bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel. Calibration Of A Bomb Calorimeter Occurs.
From www.studocu.com
2022 FA Bomb Calorimetry Jacey Scott Identification of an Unknown’s Calibration Of A Bomb Calorimeter Occurs bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. w = p δ v and δ v. Calibration Of A Bomb Calorimeter Occurs.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Calibration Of A Bomb Calorimeter Occurs a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb). constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. w = p δ v and δ v is. Calibration Of A Bomb Calorimeter Occurs.
From www.youtube.com
Bomb Calorimeter Definition, Construction, Working & Uses YouTube Calibration Of A Bomb Calorimeter Occurs So, for a constant volume, δe = q, and therefore, the bomb calorimeter. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. constant volume calorimetry, also know as bomb calorimetry, is used to measure. Calibration Of A Bomb Calorimeter Occurs.
From chem.libretexts.org
6.5 Constant Volume Calorimetry Measuring ΔU for Chemical Reactions Calibration Of A Bomb Calorimeter Occurs bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. bomb calorimetry. Calibration Of A Bomb Calorimeter Occurs.
From www.chegg.com
Solved Question Naphthalene Combustion Can Be Used To Cal... Calibration Of A Bomb Calorimeter Occurs bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimetry is used to determine the enthalpy of combustion, d combh, for hydrocarbons: bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. So, for a constant volume, δe = q, and therefore, the. Calibration Of A Bomb Calorimeter Occurs.
From www.chegg.com
Solved A chemist wants to calibrate a new bomb Calorimeter. Calibration Of A Bomb Calorimeter Occurs bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimetry is used to determine the enthalpy of combustion, d combh, for hydrocarbons: So, for a constant volume, δe = q, and therefore, the bomb calorimeter. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure. Calibration Of A Bomb Calorimeter Occurs.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calibration Of A Bomb Calorimeter Occurs w = p δ v and δ v is zero when the volume is constant. bomb calorimetry is used to determine the enthalpy of combustion, d combh, for hydrocarbons: bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. a bomb calorimeter is used to measure, under controlled conditions, the. Calibration Of A Bomb Calorimeter Occurs.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calibration Of A Bomb Calorimeter Occurs w = p δ v and δ v is zero when the volume is constant. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. bomb calorimetry is used to determine the enthalpy of combustion, d combh, for hydrocarbons: bomb calorimeters require calibration to. Calibration Of A Bomb Calorimeter Occurs.
From www.indiamart.com
Digital Bomb Calorimeter Calibration Services at best price in Nashik Calibration Of A Bomb Calorimeter Occurs bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel. Calibration Of A Bomb Calorimeter Occurs.
From www.youtube.com
How to Calculate Enthalpy Change Using a Calorimeter YouTube Calibration Of A Bomb Calorimeter Occurs bomb calorimetry is used to determine the enthalpy of combustion, d combh, for hydrocarbons: So, for a constant volume, δe = q, and therefore, the bomb calorimeter. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by. Calibration Of A Bomb Calorimeter Occurs.
From www.vrogue.co
What Is Calorimetry With Pictures vrogue.co Calibration Of A Bomb Calorimeter Occurs a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb). w = p δ v and δ v is zero when the volume is constant. bomb calorimetry is used to determine the enthalpy of combustion, d combh, for hydrocarbons: constant. Calibration Of A Bomb Calorimeter Occurs.
From giocjbkvv.blob.core.windows.net
Bomb Calorimeter Calibration at Susan Le blog Calibration Of A Bomb Calorimeter Occurs bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimetry is used to determine the enthalpy of combustion, d combh, for hydrocarbons: bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. So, for a constant volume, δe = q, and therefore, the. Calibration Of A Bomb Calorimeter Occurs.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calibration Of A Bomb Calorimeter Occurs So, for a constant volume, δe = q, and therefore, the bomb calorimeter. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. w = p δ v and. Calibration Of A Bomb Calorimeter Occurs.
From www.laboratory-equipment.com
Calorimeter Features, Styles, Types, and Differences Calibration Of A Bomb Calorimeter Occurs constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb). bomb calorimeters require calibration to determine the heat capacity. Calibration Of A Bomb Calorimeter Occurs.
From www.animalia-life.club
Calorimeter Diagram Calibration Of A Bomb Calorimeter Occurs constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. w = p δ v and δ v is zero when the volume is constant. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. a bomb calorimeter. Calibration Of A Bomb Calorimeter Occurs.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calibration Of A Bomb Calorimeter Occurs So, for a constant volume, δe = q, and therefore, the bomb calorimeter. bomb calorimetry is used to determine the enthalpy of combustion, d combh, for hydrocarbons: a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb). bomb calorimeters require calibration. Calibration Of A Bomb Calorimeter Occurs.
From www.scribd.com
Bomb Calorimeter Calibration Record PDF Calibration Of A Bomb Calorimeter Occurs a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb). So, for a constant volume, δe = q, and therefore, the bomb calorimeter. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. w =. Calibration Of A Bomb Calorimeter Occurs.
From www.academia.edu
(PDF) Bomb Calorimetry Experiment Bryle Kristiann Camarote Academia.edu Calibration Of A Bomb Calorimeter Occurs constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. bomb calorimetry is used to determine the enthalpy of combustion, d combh, for hydrocarbons: bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimeters require calibration. Calibration Of A Bomb Calorimeter Occurs.
From dxopjumlc.blob.core.windows.net
Calorimeter Calibration Calculator at Jeffery Williams blog Calibration Of A Bomb Calorimeter Occurs So, for a constant volume, δe = q, and therefore, the bomb calorimeter. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. a bomb calorimeter is used to. Calibration Of A Bomb Calorimeter Occurs.
From courses.lumenlearning.com
Calorimetry CHEM 1305 Introductory Chemistry Calibration Of A Bomb Calorimeter Occurs So, for a constant volume, δe = q, and therefore, the bomb calorimeter. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. constant volume calorimetry, also know as bomb calorimetry, is used to measure. Calibration Of A Bomb Calorimeter Occurs.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, Calibration Of A Bomb Calorimeter Occurs bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. w = p δ v and δ v is zero when the volume is constant. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted. Calibration Of A Bomb Calorimeter Occurs.
From www.chegg.com
Solved a) Calibration i. A bomb calorimeter filled with Calibration Of A Bomb Calorimeter Occurs bomb calorimetry is used to determine the enthalpy of combustion, d combh, for hydrocarbons: bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. w = p δ v and δ v is zero when the volume is. Calibration Of A Bomb Calorimeter Occurs.
From exohkbfgq.blob.core.windows.net
Calorimeter For Experiment at Lillian Bordner blog Calibration Of A Bomb Calorimeter Occurs bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. w = p δ v and. Calibration Of A Bomb Calorimeter Occurs.
From www.labrotovap.com
Laboratory Digital Automatic Metal Bomb Calorimeter Lab Instrument Calibration Of A Bomb Calorimeter Occurs constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimetry is used to determine the enthalpy of combustion, d combh, for hydrocarbons: bomb calorimeters require calibration. Calibration Of A Bomb Calorimeter Occurs.
From schoolbag.info
Image Calibration Of A Bomb Calorimeter Occurs bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimetry is used to determine the enthalpy of combustion, d combh, for hydrocarbons: So, for a constant volume, δe = q, and therefore, the. Calibration Of A Bomb Calorimeter Occurs.
From id.scribd.com
BOMB CALORIMETER CALIBRATION REPORT PDF Calibration Of A Bomb Calorimeter Occurs bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel. Calibration Of A Bomb Calorimeter Occurs.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calibration Of A Bomb Calorimeter Occurs bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. w = p δ v and δ v is zero when the volume is constant. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and. Calibration Of A Bomb Calorimeter Occurs.
From www.studocu.com
Bomb Calorimeter Problems Bomb Calorimeter Practice Problems Name Calibration Of A Bomb Calorimeter Occurs So, for a constant volume, δe = q, and therefore, the bomb calorimeter. bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. w = p δ v and δ v is zero when the volume is constant. bomb calorimetry is used to determine the enthalpy of combustion, d combh, for. Calibration Of A Bomb Calorimeter Occurs.