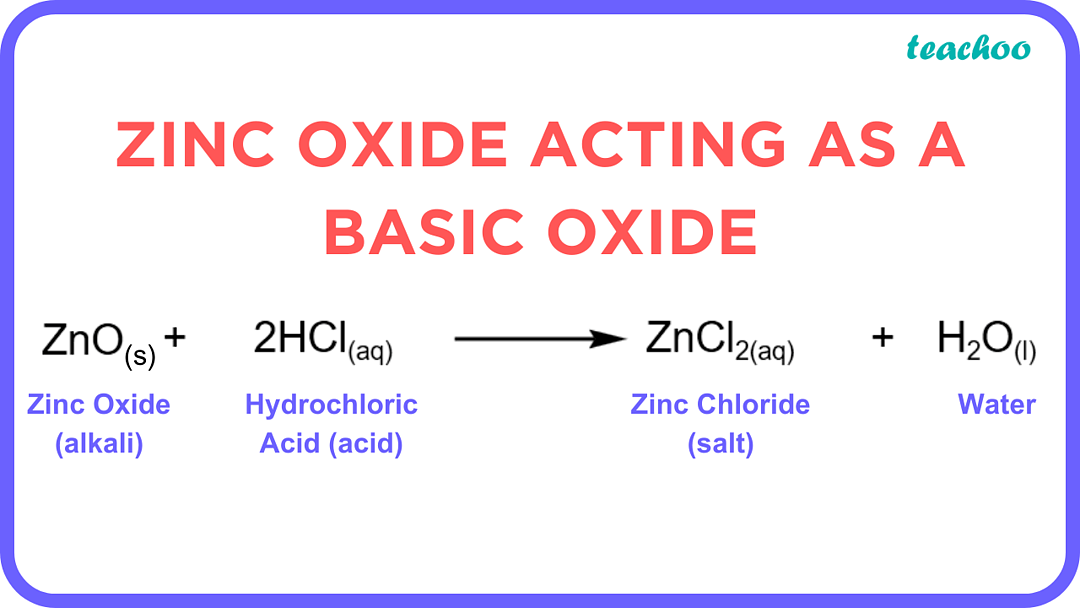
Zinc Hydroxide Amphoteric . [] in its compounds, zn 2+ ions have an electronic configuration [ar] 3d 10. It is an inorganic chemical compound that naturally exists as a rare mineral. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. Zinc hydroxide is an inorganic chemical compound with formula zn(oh) 2. Many metals (such as copper, zinc, tin, lead, aluminium, and beryllium) form amphoteric oxides or hydroxides. Amphoterism depends on the oxidation state of the oxide. As such, zn 2+ tends to have a symmetrical. Zinc hydroxide, denoted by the chemical formula zn (oh) 2, is an inorganic chemical compound that plays a vital role in many chemical reactions. It can dissolve equally in a solution of strong acid or base and is called an amphoteric.
from www.teachoo.com
It can dissolve equally in a solution of strong acid or base and is called an amphoteric. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. As such, zn 2+ tends to have a symmetrical. Zinc hydroxide is an inorganic chemical compound with formula zn(oh) 2. It is an inorganic chemical compound that naturally exists as a rare mineral. Amphoterism depends on the oxidation state of the oxide. Zinc hydroxide, denoted by the chemical formula zn (oh) 2, is an inorganic chemical compound that plays a vital role in many chemical reactions. Many metals (such as copper, zinc, tin, lead, aluminium, and beryllium) form amphoteric oxides or hydroxides. [] in its compounds, zn 2+ ions have an electronic configuration [ar] 3d 10.
What are amphoteric oxides? Give two examples Teachoo Science
Zinc Hydroxide Amphoteric It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. Amphoterism depends on the oxidation state of the oxide. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. [] in its compounds, zn 2+ ions have an electronic configuration [ar] 3d 10. It can dissolve equally in a solution of strong acid or base and is called an amphoteric. Zinc hydroxide is an inorganic chemical compound with formula zn(oh) 2. Many metals (such as copper, zinc, tin, lead, aluminium, and beryllium) form amphoteric oxides or hydroxides. Zinc hydroxide, denoted by the chemical formula zn (oh) 2, is an inorganic chemical compound that plays a vital role in many chemical reactions. It is an inorganic chemical compound that naturally exists as a rare mineral. As such, zn 2+ tends to have a symmetrical.
From www.toppr.com
Sodium Hyulum Lead oxide (? Checkuprogress! TITITI Choose the correct Zinc Hydroxide Amphoteric It is an inorganic chemical compound that naturally exists as a rare mineral. As such, zn 2+ tends to have a symmetrical. It can dissolve equally in a solution of strong acid or base and is called an amphoteric. Zinc hydroxide, denoted by the chemical formula zn (oh) 2, is an inorganic chemical compound that plays a vital role in. Zinc Hydroxide Amphoteric.
From giovvmvsw.blob.core.windows.net
Zinc Hydroxide Soluble Or Insoluble at Joy Kane blog Zinc Hydroxide Amphoteric [] in its compounds, zn 2+ ions have an electronic configuration [ar] 3d 10. It is an inorganic chemical compound that naturally exists as a rare mineral. Zinc hydroxide, denoted by the chemical formula zn (oh) 2, is an inorganic chemical compound that plays a vital role in many chemical reactions. Zinc hydroxide is an inorganic chemical compound with formula. Zinc Hydroxide Amphoteric.
From www.toppr.com
Write a balanced equation when zinc reacts with sodium hydroxide. Zinc Hydroxide Amphoteric [] in its compounds, zn 2+ ions have an electronic configuration [ar] 3d 10. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. Zinc hydroxide is an inorganic chemical compound with formula zn(oh) 2. Zinc hydroxide, denoted by the chemical formula zn (oh) 2, is an inorganic chemical compound that plays a vital role in many chemical. Zinc Hydroxide Amphoteric.
From www.slideserve.com
PPT Acid and Bases PowerPoint Presentation, free download ID6947838 Zinc Hydroxide Amphoteric As such, zn 2+ tends to have a symmetrical. Zinc hydroxide is an inorganic chemical compound with formula zn(oh) 2. Amphoterism depends on the oxidation state of the oxide. It can dissolve equally in a solution of strong acid or base and is called an amphoteric. Zinc hydroxide, denoted by the chemical formula zn (oh) 2, is an inorganic chemical. Zinc Hydroxide Amphoteric.
From www.chegg.com
Solved The text describes zinc hydroxide as an amphoteric Zinc Hydroxide Amphoteric [] in its compounds, zn 2+ ions have an electronic configuration [ar] 3d 10. Many metals (such as copper, zinc, tin, lead, aluminium, and beryllium) form amphoteric oxides or hydroxides. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. It is an inorganic chemical compound that naturally exists as a rare mineral. Zinc hydroxide is an inorganic. Zinc Hydroxide Amphoteric.
From www.indiamart.com
Zinc Hydroxide, 500g 1 Kg, Rs 70 /kilogram(s), Suraj Fine Chemicals Zinc Hydroxide Amphoteric It is an inorganic chemical compound that naturally exists as a rare mineral. Amphoterism depends on the oxidation state of the oxide. Zinc hydroxide is an inorganic chemical compound with formula zn(oh) 2. As such, zn 2+ tends to have a symmetrical. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. Zinc hydroxide, denoted by the chemical. Zinc Hydroxide Amphoteric.
From www.youtube.com
How to Balance Zn(OH)2 = ZnO + H2O (Zinc hydroxide YouTube Zinc Hydroxide Amphoteric Zinc hydroxide, denoted by the chemical formula zn (oh) 2, is an inorganic chemical compound that plays a vital role in many chemical reactions. As such, zn 2+ tends to have a symmetrical. Amphoterism depends on the oxidation state of the oxide. It can dissolve equally in a solution of strong acid or base and is called an amphoteric. []. Zinc Hydroxide Amphoteric.
From sciencenotes.org
Amphoterism Amphoteric Definition and Examples Zinc Hydroxide Amphoteric Many metals (such as copper, zinc, tin, lead, aluminium, and beryllium) form amphoteric oxides or hydroxides. Amphoterism depends on the oxidation state of the oxide. [] in its compounds, zn 2+ ions have an electronic configuration [ar] 3d 10. As such, zn 2+ tends to have a symmetrical. Zinc hydroxide is an inorganic chemical compound with formula zn(oh) 2. Zinc. Zinc Hydroxide Amphoteric.
From www.numerade.com
SOLVEDZinc hydroxide is an amphoteric substance. Write equations that Zinc Hydroxide Amphoteric Zinc hydroxide, denoted by the chemical formula zn (oh) 2, is an inorganic chemical compound that plays a vital role in many chemical reactions. Many metals (such as copper, zinc, tin, lead, aluminium, and beryllium) form amphoteric oxides or hydroxides. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. Amphoterism depends on the oxidation state of the. Zinc Hydroxide Amphoteric.
From advancedchemsys.com
Zinc Removal From Water Advanced Chemical Systems Zinc Hydroxide Amphoteric As such, zn 2+ tends to have a symmetrical. Zinc hydroxide is an inorganic chemical compound with formula zn(oh) 2. Amphoterism depends on the oxidation state of the oxide. [] in its compounds, zn 2+ ions have an electronic configuration [ar] 3d 10. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. Zinc hydroxide, denoted by the. Zinc Hydroxide Amphoteric.
From aotaixincailiao.en.made-in-china.com
Aluminium Hydroxide CAS No. 21645512 Used as Fire Retardant Filler Zinc Hydroxide Amphoteric As such, zn 2+ tends to have a symmetrical. [] in its compounds, zn 2+ ions have an electronic configuration [ar] 3d 10. Zinc hydroxide is an inorganic chemical compound with formula zn(oh) 2. It is an inorganic chemical compound that naturally exists as a rare mineral. Many metals (such as copper, zinc, tin, lead, aluminium, and beryllium) form amphoteric. Zinc Hydroxide Amphoteric.
From www.indiamart.com
Zinc Hydroxide Powder at Rs 148/kg in Surat ID 23244970588 Zinc Hydroxide Amphoteric It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. As such, zn 2+ tends to have a symmetrical. Many metals (such as copper, zinc, tin, lead, aluminium, and beryllium) form amphoteric oxides or hydroxides. Amphoterism depends on the oxidation state of the oxide. It is an inorganic chemical compound that naturally exists as a rare mineral. Zinc. Zinc Hydroxide Amphoteric.
From www.aashichem.in
Pharmaceutical Tablets Zinc Hydroxide Manufacturer from Surat Zinc Hydroxide Amphoteric [] in its compounds, zn 2+ ions have an electronic configuration [ar] 3d 10. Many metals (such as copper, zinc, tin, lead, aluminium, and beryllium) form amphoteric oxides or hydroxides. Amphoterism depends on the oxidation state of the oxide. It can dissolve equally in a solution of strong acid or base and is called an amphoteric. It occurs naturally in. Zinc Hydroxide Amphoteric.
From www.slideserve.com
PPT Complex Ion Equilibria PowerPoint Presentation, free download Zinc Hydroxide Amphoteric It can dissolve equally in a solution of strong acid or base and is called an amphoteric. Amphoterism depends on the oxidation state of the oxide. Many metals (such as copper, zinc, tin, lead, aluminium, and beryllium) form amphoteric oxides or hydroxides. [] in its compounds, zn 2+ ions have an electronic configuration [ar] 3d 10. Zinc hydroxide is an. Zinc Hydroxide Amphoteric.
From www.chegg.com
Solved The text describes zinc hydroxide as an amphoteric Zinc Hydroxide Amphoteric It can dissolve equally in a solution of strong acid or base and is called an amphoteric. Zinc hydroxide is an inorganic chemical compound with formula zn(oh) 2. As such, zn 2+ tends to have a symmetrical. [] in its compounds, zn 2+ ions have an electronic configuration [ar] 3d 10. Amphoterism depends on the oxidation state of the oxide.. Zinc Hydroxide Amphoteric.
From woelen.homescience.net
Science made alive Chemistry/Solutions Zinc Hydroxide Amphoteric It is an inorganic chemical compound that naturally exists as a rare mineral. Zinc hydroxide is an inorganic chemical compound with formula zn(oh) 2. It can dissolve equally in a solution of strong acid or base and is called an amphoteric. Zinc hydroxide, denoted by the chemical formula zn (oh) 2, is an inorganic chemical compound that plays a vital. Zinc Hydroxide Amphoteric.
From fineartamerica.com
Zinc Hydroxide Precipitate Photograph by Andrew Lambert Photography Zinc Hydroxide Amphoteric As such, zn 2+ tends to have a symmetrical. [] in its compounds, zn 2+ ions have an electronic configuration [ar] 3d 10. Many metals (such as copper, zinc, tin, lead, aluminium, and beryllium) form amphoteric oxides or hydroxides. Zinc hydroxide, denoted by the chemical formula zn (oh) 2, is an inorganic chemical compound that plays a vital role in. Zinc Hydroxide Amphoteric.
From ar.inspiredpencil.com
Zinc Hydroxide Zinc Hydroxide Amphoteric It is an inorganic chemical compound that naturally exists as a rare mineral. Amphoterism depends on the oxidation state of the oxide. As such, zn 2+ tends to have a symmetrical. It can dissolve equally in a solution of strong acid or base and is called an amphoteric. [] in its compounds, zn 2+ ions have an electronic configuration [ar]. Zinc Hydroxide Amphoteric.
From www.teachoo.com
What are amphoteric oxides? Give two examples Teachoo Science Zinc Hydroxide Amphoteric Amphoterism depends on the oxidation state of the oxide. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. As such, zn 2+ tends to have a symmetrical. Many metals (such as copper, zinc, tin, lead, aluminium, and beryllium) form amphoteric oxides or hydroxides. It is an inorganic chemical compound that naturally exists as a rare mineral. []. Zinc Hydroxide Amphoteric.
From www.numerade.com
SOLVEDZinc hydroxide is an amphoteric substance. Write equations that Zinc Hydroxide Amphoteric As such, zn 2+ tends to have a symmetrical. Amphoterism depends on the oxidation state of the oxide. Zinc hydroxide, denoted by the chemical formula zn (oh) 2, is an inorganic chemical compound that plays a vital role in many chemical reactions. Many metals (such as copper, zinc, tin, lead, aluminium, and beryllium) form amphoteric oxides or hydroxides. Zinc hydroxide. Zinc Hydroxide Amphoteric.
From ar.inspiredpencil.com
Amphoteric Elements Zinc Hydroxide Amphoteric Amphoterism depends on the oxidation state of the oxide. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. As such, zn 2+ tends to have a symmetrical. [] in its compounds, zn 2+ ions have an electronic configuration [ar] 3d 10. Zinc hydroxide, denoted by the chemical formula zn (oh) 2, is an inorganic chemical compound that. Zinc Hydroxide Amphoteric.
From brainly.in
What are amphoteric oxides?Is water a amphoteric oxide.Why or why not Zinc Hydroxide Amphoteric Amphoterism depends on the oxidation state of the oxide. Zinc hydroxide is an inorganic chemical compound with formula zn(oh) 2. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. It can dissolve equally in a solution of strong acid or base and is called an amphoteric. It is an inorganic chemical compound that naturally exists as a. Zinc Hydroxide Amphoteric.
From www.toppr.com
The pair of amphoteric hydroxide is a. Al(OH)3, LiOH b. Be(OH)2, Mg Zinc Hydroxide Amphoteric Amphoterism depends on the oxidation state of the oxide. It is an inorganic chemical compound that naturally exists as a rare mineral. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. Zinc hydroxide is an inorganic chemical compound with formula zn(oh) 2. It can dissolve equally in a solution of strong acid or base and is called. Zinc Hydroxide Amphoteric.
From www.indiamart.com
Zinc Hydroxide Powder at Rs 148/kg in Surat ID 23244970588 Zinc Hydroxide Amphoteric [] in its compounds, zn 2+ ions have an electronic configuration [ar] 3d 10. It can dissolve equally in a solution of strong acid or base and is called an amphoteric. Amphoterism depends on the oxidation state of the oxide. Zinc hydroxide is an inorganic chemical compound with formula zn(oh) 2. As such, zn 2+ tends to have a symmetrical.. Zinc Hydroxide Amphoteric.
From www.toppr.com
Which elements of group 13 form amphoteric hydroxides? Zinc Hydroxide Amphoteric Amphoterism depends on the oxidation state of the oxide. It is an inorganic chemical compound that naturally exists as a rare mineral. [] in its compounds, zn 2+ ions have an electronic configuration [ar] 3d 10. Zinc hydroxide is an inorganic chemical compound with formula zn(oh) 2. Zinc hydroxide, denoted by the chemical formula zn (oh) 2, is an inorganic. Zinc Hydroxide Amphoteric.
From www.coursehero.com
[Solved] . 1. Zinc oxide (ZnO) is considered as amphoteric because it Zinc Hydroxide Amphoteric Zinc hydroxide is an inorganic chemical compound with formula zn(oh) 2. As such, zn 2+ tends to have a symmetrical. It can dissolve equally in a solution of strong acid or base and is called an amphoteric. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. Amphoterism depends on the oxidation state of the oxide. Zinc hydroxide,. Zinc Hydroxide Amphoteric.
From www.chegg.com
Solved Zinc hydroxide (Zn(OH)2) is an amphoteric compound. Zinc Hydroxide Amphoteric As such, zn 2+ tends to have a symmetrical. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. [] in its compounds, zn 2+ ions have an electronic configuration [ar] 3d 10. Amphoterism depends on the oxidation state of the oxide. It can dissolve equally in a solution of strong acid or base and is called an. Zinc Hydroxide Amphoteric.
From www.fishersci.com
Zinc Hydroxide, MP Biomedicals, Quantity 100 g Fisher Scientific Zinc Hydroxide Amphoteric Zinc hydroxide, denoted by the chemical formula zn (oh) 2, is an inorganic chemical compound that plays a vital role in many chemical reactions. Many metals (such as copper, zinc, tin, lead, aluminium, and beryllium) form amphoteric oxides or hydroxides. [] in its compounds, zn 2+ ions have an electronic configuration [ar] 3d 10. Amphoterism depends on the oxidation state. Zinc Hydroxide Amphoteric.
From www.indiamart.com
Zinc Hydroxide, Packaging Type Bag at best price in Vapi ID 1711557962 Zinc Hydroxide Amphoteric Many metals (such as copper, zinc, tin, lead, aluminium, and beryllium) form amphoteric oxides or hydroxides. As such, zn 2+ tends to have a symmetrical. Zinc hydroxide is an inorganic chemical compound with formula zn(oh) 2. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. Amphoterism depends on the oxidation state of the oxide. It can dissolve. Zinc Hydroxide Amphoteric.
From brainly.in
What are amphoteric oxides. Explain with the help of chemical reactions Zinc Hydroxide Amphoteric Amphoterism depends on the oxidation state of the oxide. Zinc hydroxide is an inorganic chemical compound with formula zn(oh) 2. It is an inorganic chemical compound that naturally exists as a rare mineral. [] in its compounds, zn 2+ ions have an electronic configuration [ar] 3d 10. Zinc hydroxide, denoted by the chemical formula zn (oh) 2, is an inorganic. Zinc Hydroxide Amphoteric.
From giovvmvsw.blob.core.windows.net
Zinc Hydroxide Soluble Or Insoluble at Joy Kane blog Zinc Hydroxide Amphoteric As such, zn 2+ tends to have a symmetrical. [] in its compounds, zn 2+ ions have an electronic configuration [ar] 3d 10. Zinc hydroxide is an inorganic chemical compound with formula zn(oh) 2. Many metals (such as copper, zinc, tin, lead, aluminium, and beryllium) form amphoteric oxides or hydroxides. Amphoterism depends on the oxidation state of the oxide. Zinc. Zinc Hydroxide Amphoteric.
From brainly.in
some metallic oxide is amphoteric in nature justify with suitable Zinc Hydroxide Amphoteric It is an inorganic chemical compound that naturally exists as a rare mineral. As such, zn 2+ tends to have a symmetrical. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. Many metals (such as copper, zinc, tin, lead, aluminium, and beryllium) form amphoteric oxides or hydroxides. Amphoterism depends on the oxidation state of the oxide. Zinc. Zinc Hydroxide Amphoteric.
From whatsinsight.org
Zinc Hydroxide Structure, Properties, and Uses What's Insight Zinc Hydroxide Amphoteric Zinc hydroxide, denoted by the chemical formula zn (oh) 2, is an inorganic chemical compound that plays a vital role in many chemical reactions. As such, zn 2+ tends to have a symmetrical. Amphoterism depends on the oxidation state of the oxide. It is an inorganic chemical compound that naturally exists as a rare mineral. It occurs naturally in three. Zinc Hydroxide Amphoteric.
From www.toppr.com
Sodium Hyulum Lead oxide (? Checkuprogress! TITITI Choose the correct Zinc Hydroxide Amphoteric It can dissolve equally in a solution of strong acid or base and is called an amphoteric. Zinc hydroxide, denoted by the chemical formula zn (oh) 2, is an inorganic chemical compound that plays a vital role in many chemical reactions. Amphoterism depends on the oxidation state of the oxide. [] in its compounds, zn 2+ ions have an electronic. Zinc Hydroxide Amphoteric.
From brainly.in
Explain the chemical reaction why is zinc oxide amphoteric in nature Zinc Hydroxide Amphoteric Zinc hydroxide is an inorganic chemical compound with formula zn(oh) 2. Zinc hydroxide, denoted by the chemical formula zn (oh) 2, is an inorganic chemical compound that plays a vital role in many chemical reactions. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. As such, zn 2+ tends to have a symmetrical. It can dissolve equally. Zinc Hydroxide Amphoteric.