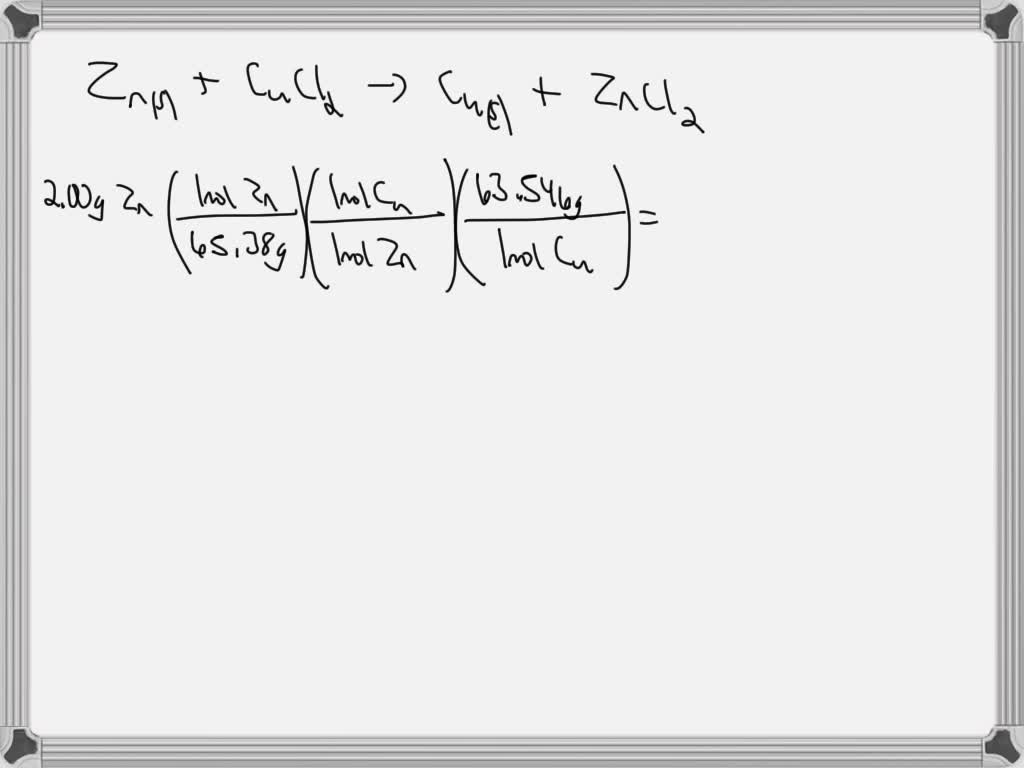Copper(Ii) Chloride Practice Problems . To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves. Limiting reagent & percent yield practice problems. An hydrate of copper (ii) chloride has the formula cucl 2 ⋅ xh 2 o. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium. From these data, determine the value of x and write the complete formula for hydrated copper (ii) chloride. Number of \(cu^{2+}\) ions in 52 l of 2.3 m copper (ii). The water in a 3.41 g sample of the hydrate was driven off by. When you work out how much sodium chloride can be made with 15 grams of. To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves. The number of moles and the mass (in kg) of copper(ii) carbonate needed to decompose in order to produce 1.500 kg of copper(ii) oxide,. 1) determine the mass of the. Volume in liters of 2.26 m potassium hydroxide that contains 8.42 g of solute;
from www.numerade.com
Limiting reagent & percent yield practice problems. Volume in liters of 2.26 m potassium hydroxide that contains 8.42 g of solute; When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium. 1) determine the mass of the. An hydrate of copper (ii) chloride has the formula cucl 2 ⋅ xh 2 o. When you work out how much sodium chloride can be made with 15 grams of. Number of \(cu^{2+}\) ions in 52 l of 2.3 m copper (ii). From these data, determine the value of x and write the complete formula for hydrated copper (ii) chloride. To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves. The number of moles and the mass (in kg) of copper(ii) carbonate needed to decompose in order to produce 1.500 kg of copper(ii) oxide,.
SOLVED A strip of zinc metal immersed in 207 mL of an aqueous solution
Copper(Ii) Chloride Practice Problems 1) determine the mass of the. From these data, determine the value of x and write the complete formula for hydrated copper (ii) chloride. To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves. An hydrate of copper (ii) chloride has the formula cucl 2 ⋅ xh 2 o. Volume in liters of 2.26 m potassium hydroxide that contains 8.42 g of solute; The number of moles and the mass (in kg) of copper(ii) carbonate needed to decompose in order to produce 1.500 kg of copper(ii) oxide,. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium. The water in a 3.41 g sample of the hydrate was driven off by. To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves. 1) determine the mass of the. Limiting reagent & percent yield practice problems. Number of \(cu^{2+}\) ions in 52 l of 2.3 m copper (ii). When you work out how much sodium chloride can be made with 15 grams of.
From www.youtube.com
The electrolysis of copper (II) chloride. YouTube Copper(Ii) Chloride Practice Problems Number of \(cu^{2+}\) ions in 52 l of 2.3 m copper (ii). To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves. When you work out how much sodium chloride can be made with 15 grams of. The water in a 3.41 g sample of the hydrate was driven off by.. Copper(Ii) Chloride Practice Problems.
From www.numerade.com
SOLVED a. Write a balanced molecular equation for the reaction of Copper(Ii) Chloride Practice Problems 1) determine the mass of the. When you work out how much sodium chloride can be made with 15 grams of. Limiting reagent & percent yield practice problems. The water in a 3.41 g sample of the hydrate was driven off by. Volume in liters of 2.26 m potassium hydroxide that contains 8.42 g of solute; To solve this problem. Copper(Ii) Chloride Practice Problems.
From mackenzie-chapter.blogspot.com
Aluminum Copper Ii Chloride Reaction Lab Answers 50+ Pages Summary Doc Copper(Ii) Chloride Practice Problems Limiting reagent & percent yield practice problems. From these data, determine the value of x and write the complete formula for hydrated copper (ii) chloride. 1) determine the mass of the. The number of moles and the mass (in kg) of copper(ii) carbonate needed to decompose in order to produce 1.500 kg of copper(ii) oxide,. Volume in liters of 2.26. Copper(Ii) Chloride Practice Problems.
From www.chegg.com
Solved A solution of copper(II) chloride reacts with Copper(Ii) Chloride Practice Problems The number of moles and the mass (in kg) of copper(ii) carbonate needed to decompose in order to produce 1.500 kg of copper(ii) oxide,. To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves. 1) determine the mass of the. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate. Copper(Ii) Chloride Practice Problems.
From www.numerade.com
SOLVED Consider the following reaction When copper (II) chloride Copper(Ii) Chloride Practice Problems The number of moles and the mass (in kg) of copper(ii) carbonate needed to decompose in order to produce 1.500 kg of copper(ii) oxide,. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium. Number of \(cu^{2+}\) ions in 52 l of 2.3 m copper (ii). When you work out how much sodium chloride can be made. Copper(Ii) Chloride Practice Problems.
From www.numerade.com
SOLVEDA concentrated aqueous copper(II) chloride solution is bright Copper(Ii) Chloride Practice Problems Number of \(cu^{2+}\) ions in 52 l of 2.3 m copper (ii). Volume in liters of 2.26 m potassium hydroxide that contains 8.42 g of solute; Limiting reagent & percent yield practice problems. The water in a 3.41 g sample of the hydrate was driven off by. When you work out how much sodium chloride can be made with 15. Copper(Ii) Chloride Practice Problems.
From www.chegg.com
Solved Limiting Reagent and Percent Yield When copper (II) Copper(Ii) Chloride Practice Problems When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium. Volume in liters of 2.26 m potassium hydroxide that contains 8.42 g of solute; Limiting reagent & percent yield practice problems. When you work out how much sodium chloride can be made with 15 grams of. The number of moles and the mass (in kg) of copper(ii). Copper(Ii) Chloride Practice Problems.
From www.numerade.com
Write the balanced molecular equation for each of the following Copper(Ii) Chloride Practice Problems When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium. The water in a 3.41 g sample of the hydrate was driven off by. To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves. To solve this problem determine how much sodium chloride can be made from each. Copper(Ii) Chloride Practice Problems.
From www.numerade.com
SOLVED A solution is prepared by dissolving 63.8 grams of copper (II Copper(Ii) Chloride Practice Problems When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium. The number of moles and the mass (in kg) of copper(ii) carbonate needed to decompose in order to produce 1.500 kg of copper(ii) oxide,. From these data, determine the value of x and write the complete formula for hydrated copper (ii) chloride. Volume in liters of 2.26. Copper(Ii) Chloride Practice Problems.
From www.chegg.com
Solved DATA AND CALCULATIONS A. SYNTHESIS OF COPPER (II) Copper(Ii) Chloride Practice Problems Number of \(cu^{2+}\) ions in 52 l of 2.3 m copper (ii). To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves. From these data, determine the value of x and write the complete formula for hydrated copper (ii) chloride. Volume in liters of 2.26 m potassium hydroxide that contains 8.42. Copper(Ii) Chloride Practice Problems.
From www.numerade.com
SOLVED 6. Copper(II) oxide reacts with hydrochloric acid to produce Copper(Ii) Chloride Practice Problems Volume in liters of 2.26 m potassium hydroxide that contains 8.42 g of solute; The number of moles and the mass (in kg) of copper(ii) carbonate needed to decompose in order to produce 1.500 kg of copper(ii) oxide,. An hydrate of copper (ii) chloride has the formula cucl 2 ⋅ xh 2 o. When copper (ii) chloride reacts with sodium. Copper(Ii) Chloride Practice Problems.
From www.chegg.com
Solved Mass of copper (II) chloride sample (by subtraction) Copper(Ii) Chloride Practice Problems The number of moles and the mass (in kg) of copper(ii) carbonate needed to decompose in order to produce 1.500 kg of copper(ii) oxide,. 1) determine the mass of the. Limiting reagent & percent yield practice problems. To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves. When copper (ii) chloride. Copper(Ii) Chloride Practice Problems.
From www.chegg.com
Solved Copper (II) Chloride and Aluminum Reaction (done Copper(Ii) Chloride Practice Problems Volume in liters of 2.26 m potassium hydroxide that contains 8.42 g of solute; When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium. Limiting reagent & percent yield practice problems. 1) determine the mass of the. Number of \(cu^{2+}\) ions in 52 l of 2.3 m copper (ii). When you work out how much sodium chloride. Copper(Ii) Chloride Practice Problems.
From studylib.net
Reaction of Copper (II) Chloride and Aluminum Copper(Ii) Chloride Practice Problems An hydrate of copper (ii) chloride has the formula cucl 2 ⋅ xh 2 o. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium. To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves. Number of \(cu^{2+}\) ions in 52 l of 2.3 m copper (ii). The. Copper(Ii) Chloride Practice Problems.
From www.numerade.com
SOLVED A strip of zinc metal immersed in 207 mL of an aqueous solution Copper(Ii) Chloride Practice Problems Volume in liters of 2.26 m potassium hydroxide that contains 8.42 g of solute; When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium. An hydrate of copper (ii) chloride has the formula cucl 2 ⋅ xh 2 o. Limiting reagent & percent yield practice problems. From these data, determine the value of x and write the. Copper(Ii) Chloride Practice Problems.
From www.researchgate.net
(PDF) Underglazing with copper(II)chloride Copper(Ii) Chloride Practice Problems The water in a 3.41 g sample of the hydrate was driven off by. To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves. Volume in liters of 2.26 m potassium hydroxide that contains 8.42 g of solute; When you work out how much sodium chloride can be made with 15. Copper(Ii) Chloride Practice Problems.
From www.chegg.com
Solved If 15 grams of copper (II) chloride react with 20 Copper(Ii) Chloride Practice Problems To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves. The number of moles and the mass (in kg) of copper(ii) carbonate needed to decompose in order to produce 1.500 kg of copper(ii) oxide,. The water in a 3.41 g sample of the hydrate was driven off by. 1) determine the. Copper(Ii) Chloride Practice Problems.
From www.youtube.com
Nails in Copper (II) Chloride (Explained with chemical formulas) YouTube Copper(Ii) Chloride Practice Problems Volume in liters of 2.26 m potassium hydroxide that contains 8.42 g of solute; When you work out how much sodium chloride can be made with 15 grams of. To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves. The water in a 3.41 g sample of the hydrate was driven. Copper(Ii) Chloride Practice Problems.
From www.chegg.com
Solved ANALYSIS 1. How many grams of copper(II) chloride Copper(Ii) Chloride Practice Problems To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves. To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium. Limiting reagent & percent yield practice problems. An hydrate. Copper(Ii) Chloride Practice Problems.
From www.chegg.com
Solved F. Other Reactions 14. Copper(II)chloride and water Copper(Ii) Chloride Practice Problems The water in a 3.41 g sample of the hydrate was driven off by. Number of \(cu^{2+}\) ions in 52 l of 2.3 m copper (ii). An hydrate of copper (ii) chloride has the formula cucl 2 ⋅ xh 2 o. To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves.. Copper(Ii) Chloride Practice Problems.
From www.chegg.com
Solved Limiting Reagent and Percent Yield Worksheet Name_ Copper(Ii) Chloride Practice Problems Number of \(cu^{2+}\) ions in 52 l of 2.3 m copper (ii). 1) determine the mass of the. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium. To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves. The water in a 3.41 g sample of the hydrate. Copper(Ii) Chloride Practice Problems.
From www.echemi.com
How can you find the concentration of copper(II) ions in a solution of Copper(Ii) Chloride Practice Problems Limiting reagent & percent yield practice problems. From these data, determine the value of x and write the complete formula for hydrated copper (ii) chloride. An hydrate of copper (ii) chloride has the formula cucl 2 ⋅ xh 2 o. The number of moles and the mass (in kg) of copper(ii) carbonate needed to decompose in order to produce 1.500. Copper(Ii) Chloride Practice Problems.
From www.numerade.com
SOLVED 1. When copper (II) chloride reacts with sodium nitrate, copper Copper(Ii) Chloride Practice Problems An hydrate of copper (ii) chloride has the formula cucl 2 ⋅ xh 2 o. 1) determine the mass of the. From these data, determine the value of x and write the complete formula for hydrated copper (ii) chloride. The water in a 3.41 g sample of the hydrate was driven off by. Volume in liters of 2.26 m potassium. Copper(Ii) Chloride Practice Problems.
From www.coursehero.com
[Solved] 12.Calculate the solubility of copper (II) chloride CuClz ksp Copper(Ii) Chloride Practice Problems From these data, determine the value of x and write the complete formula for hydrated copper (ii) chloride. To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves. Number of \(cu^{2+}\) ions in 52 l of 2.3 m copper (ii). Volume in liters of 2.26 m potassium hydroxide that contains 8.42. Copper(Ii) Chloride Practice Problems.
From www.chegg.com
Task 3. Empirical formula for copper (II) chloride Copper(Ii) Chloride Practice Problems Volume in liters of 2.26 m potassium hydroxide that contains 8.42 g of solute; When you work out how much sodium chloride can be made with 15 grams of. The water in a 3.41 g sample of the hydrate was driven off by. Limiting reagent & percent yield practice problems. Number of \(cu^{2+}\) ions in 52 l of 2.3 m. Copper(Ii) Chloride Practice Problems.
From www.chegg.com
Solved When copper (II) chloride reacts with sodium nitrate, Copper(Ii) Chloride Practice Problems From these data, determine the value of x and write the complete formula for hydrated copper (ii) chloride. To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium. When you work out how much sodium chloride can be. Copper(Ii) Chloride Practice Problems.
From www.chegg.com
Solved How many moles of Aluminum and copper (II) chloride Copper(Ii) Chloride Practice Problems 1) determine the mass of the. From these data, determine the value of x and write the complete formula for hydrated copper (ii) chloride. When you work out how much sodium chloride can be made with 15 grams of. Limiting reagent & percent yield practice problems. The water in a 3.41 g sample of the hydrate was driven off by.. Copper(Ii) Chloride Practice Problems.
From www.chegg.com
Solved 4. The formula weight of copper(II) chloride is g. D) Copper(Ii) Chloride Practice Problems To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves. The water in a 3.41 g sample of the hydrate was driven off by. The number of moles and the mass (in kg) of copper(ii) carbonate needed to decompose in order to produce 1.500 kg of copper(ii) oxide,. Number of \(cu^{2+}\). Copper(Ii) Chloride Practice Problems.
From www.numerade.com
SOLVED 1. When copper(II) chloride reacts with sodium nitrate, copper Copper(Ii) Chloride Practice Problems When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium. Number of \(cu^{2+}\) ions in 52 l of 2.3 m copper (ii). Limiting reagent & percent yield practice problems. The water in a 3.41 g sample of the hydrate was driven off by. From these data, determine the value of x and write the complete formula for. Copper(Ii) Chloride Practice Problems.
From www.chegg.com
Solved Part A Copper(II) Chloride Express Your Answer As Copper(Ii) Chloride Practice Problems Limiting reagent & percent yield practice problems. From these data, determine the value of x and write the complete formula for hydrated copper (ii) chloride. The number of moles and the mass (in kg) of copper(ii) carbonate needed to decompose in order to produce 1.500 kg of copper(ii) oxide,. Number of \(cu^{2+}\) ions in 52 l of 2.3 m copper. Copper(Ii) Chloride Practice Problems.
From studylib.net
A Reaction between Copper II Chloride and Copper(Ii) Chloride Practice Problems Number of \(cu^{2+}\) ions in 52 l of 2.3 m copper (ii). To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves. 1) determine the mass of the. Limiting reagent & percent yield practice problems. To solve this problem determine how much sodium chloride can be made from each of the. Copper(Ii) Chloride Practice Problems.
From www.chegg.com
Solved Mole Ratios Copper(II) chloride (CuCl,; 0.98 g) was Copper(Ii) Chloride Practice Problems 1) determine the mass of the. The water in a 3.41 g sample of the hydrate was driven off by. When you work out how much sodium chloride can be made with 15 grams of. An hydrate of copper (ii) chloride has the formula cucl 2 ⋅ xh 2 o. When copper (ii) chloride reacts with sodium nitrate, copper (ii). Copper(Ii) Chloride Practice Problems.
From testbook.com
Copper (II) Chloride Formula Structure, Properties and Uses Copper(Ii) Chloride Practice Problems The water in a 3.41 g sample of the hydrate was driven off by. To solve this problem determine how much sodium chloride can be made from each of the reagents by themselves. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium. To solve this problem determine how much sodium chloride can be made from each. Copper(Ii) Chloride Practice Problems.
From www.youtube.com
Copper and Copper II Chloride YouTube Copper(Ii) Chloride Practice Problems Number of \(cu^{2+}\) ions in 52 l of 2.3 m copper (ii). The number of moles and the mass (in kg) of copper(ii) carbonate needed to decompose in order to produce 1.500 kg of copper(ii) oxide,. When you work out how much sodium chloride can be made with 15 grams of. To solve this problem determine how much sodium chloride. Copper(Ii) Chloride Practice Problems.
From www.numerade.com
SOLVED If 18.7 grams of copper (II) chloride react with 15.0 grams of Copper(Ii) Chloride Practice Problems The number of moles and the mass (in kg) of copper(ii) carbonate needed to decompose in order to produce 1.500 kg of copper(ii) oxide,. An hydrate of copper (ii) chloride has the formula cucl 2 ⋅ xh 2 o. Number of \(cu^{2+}\) ions in 52 l of 2.3 m copper (ii). 1) determine the mass of the. Volume in liters. Copper(Ii) Chloride Practice Problems.