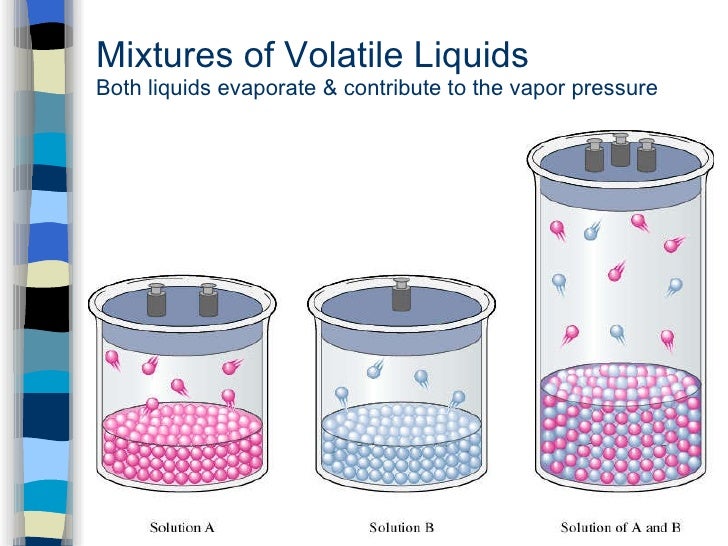
Volatile Liquid Meaning . Learn how the vapor pressure of a liquid depends on temperature and intermolecular forces. A volatile substance is one that vaporizes readily, meaning it has a high vapor pressure at a given temperature. Volatile substances are those that readily vaporize or sublimate in air. Volatile liquids are liquids that transform easily. Volatility is essentially the tendency of a liquid to evaporate. Learn about the types, properties, and applications of volatile substances in chemistry. A measure of volatility is the vapor pressure. So a highly volatile liquid would show a great tendency to evaporate. Learn how volatility is measured by vapor pressure and boiling point, and how it affects. A liquid's atmospheric pressure boiling point corresponds. It is a measure of the tendency of molecules and atoms to escape from a liquid or a solid. Explore the concepts of evaporation, condensation, and dynamic equilibrium with. A liquid that evaporates readily at normal temperature is known as a volatile liquid.
from www.slideshare.net
Learn about the types, properties, and applications of volatile substances in chemistry. A measure of volatility is the vapor pressure. Learn how volatility is measured by vapor pressure and boiling point, and how it affects. It is a measure of the tendency of molecules and atoms to escape from a liquid or a solid. A liquid that evaporates readily at normal temperature is known as a volatile liquid. A liquid's atmospheric pressure boiling point corresponds. Volatility is essentially the tendency of a liquid to evaporate. Volatile substances are those that readily vaporize or sublimate in air. Volatile liquids are liquids that transform easily. Explore the concepts of evaporation, condensation, and dynamic equilibrium with.
Solutions Powerpoint
Volatile Liquid Meaning It is a measure of the tendency of molecules and atoms to escape from a liquid or a solid. A measure of volatility is the vapor pressure. Learn how the vapor pressure of a liquid depends on temperature and intermolecular forces. Explore the concepts of evaporation, condensation, and dynamic equilibrium with. It is a measure of the tendency of molecules and atoms to escape from a liquid or a solid. A liquid that evaporates readily at normal temperature is known as a volatile liquid. Learn about the types, properties, and applications of volatile substances in chemistry. A volatile substance is one that vaporizes readily, meaning it has a high vapor pressure at a given temperature. A liquid's atmospheric pressure boiling point corresponds. Volatility is essentially the tendency of a liquid to evaporate. Learn how volatility is measured by vapor pressure and boiling point, and how it affects. So a highly volatile liquid would show a great tendency to evaporate. Volatile liquids are liquids that transform easily. Volatile substances are those that readily vaporize or sublimate in air.
From www.youtube.com
Separation of Volatile liquid solid mixture by Distillation; BSc Volatile Liquid Meaning A liquid's atmospheric pressure boiling point corresponds. Learn about the types, properties, and applications of volatile substances in chemistry. A liquid that evaporates readily at normal temperature is known as a volatile liquid. Learn how the vapor pressure of a liquid depends on temperature and intermolecular forces. So a highly volatile liquid would show a great tendency to evaporate. Explore. Volatile Liquid Meaning.
From worldnewlive.com
Which Is A Volatile Liquid? Mastery Wiki Volatile Liquid Meaning Volatile liquids are liquids that transform easily. A liquid that evaporates readily at normal temperature is known as a volatile liquid. So a highly volatile liquid would show a great tendency to evaporate. Learn how volatility is measured by vapor pressure and boiling point, and how it affects. Volatility is essentially the tendency of a liquid to evaporate. It is. Volatile Liquid Meaning.
From www.youtube.com
Raoult's Law for volatile liquids( logical and easy way to understand Volatile Liquid Meaning A volatile substance is one that vaporizes readily, meaning it has a high vapor pressure at a given temperature. Explore the concepts of evaporation, condensation, and dynamic equilibrium with. It is a measure of the tendency of molecules and atoms to escape from a liquid or a solid. A liquid that evaporates readily at normal temperature is known as a. Volatile Liquid Meaning.
From www.slideserve.com
PPT Solution Properties PowerPoint Presentation, free download ID Volatile Liquid Meaning Volatile substances are those that readily vaporize or sublimate in air. A volatile substance is one that vaporizes readily, meaning it has a high vapor pressure at a given temperature. So a highly volatile liquid would show a great tendency to evaporate. Learn how the vapor pressure of a liquid depends on temperature and intermolecular forces. Volatile liquids are liquids. Volatile Liquid Meaning.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID59727 Volatile Liquid Meaning Learn about the types, properties, and applications of volatile substances in chemistry. Volatile liquids are liquids that transform easily. It is a measure of the tendency of molecules and atoms to escape from a liquid or a solid. Volatile substances are those that readily vaporize or sublimate in air. A liquid's atmospheric pressure boiling point corresponds. A volatile substance is. Volatile Liquid Meaning.
From www.youtube.com
Volatility (chemistry) YouTube Volatile Liquid Meaning Volatile liquids are liquids that transform easily. Learn how the vapor pressure of a liquid depends on temperature and intermolecular forces. A volatile substance is one that vaporizes readily, meaning it has a high vapor pressure at a given temperature. It is a measure of the tendency of molecules and atoms to escape from a liquid or a solid. Volatility. Volatile Liquid Meaning.
From chemistryskills.com
Volatile Chemistry Chemistry Skills Volatile Liquid Meaning A liquid's atmospheric pressure boiling point corresponds. Learn how the vapor pressure of a liquid depends on temperature and intermolecular forces. Explore the concepts of evaporation, condensation, and dynamic equilibrium with. Learn about the types, properties, and applications of volatile substances in chemistry. A volatile substance is one that vaporizes readily, meaning it has a high vapor pressure at a. Volatile Liquid Meaning.
From www.hamiltoncompany.com
Tips to Accurately Pipette Volatile Liquids Hamilton Volatile Liquid Meaning Learn how the vapor pressure of a liquid depends on temperature and intermolecular forces. Volatile substances are those that readily vaporize or sublimate in air. A liquid's atmospheric pressure boiling point corresponds. Volatile liquids are liquids that transform easily. A liquid that evaporates readily at normal temperature is known as a volatile liquid. So a highly volatile liquid would show. Volatile Liquid Meaning.
From www.slideserve.com
PPT Boiling Points Distillations PowerPoint Presentation, free Volatile Liquid Meaning Learn how the vapor pressure of a liquid depends on temperature and intermolecular forces. Volatile liquids are liquids that transform easily. Volatile substances are those that readily vaporize or sublimate in air. A liquid that evaporates readily at normal temperature is known as a volatile liquid. Learn how volatility is measured by vapor pressure and boiling point, and how it. Volatile Liquid Meaning.
From www.thoughtco.com
Chemistry Glossary Definition of Volatile Volatile Liquid Meaning Learn how the vapor pressure of a liquid depends on temperature and intermolecular forces. Volatility is essentially the tendency of a liquid to evaporate. It is a measure of the tendency of molecules and atoms to escape from a liquid or a solid. Learn about the types, properties, and applications of volatile substances in chemistry. So a highly volatile liquid. Volatile Liquid Meaning.
From studylib.net
MM of a Volatile Liquid Volatile Liquid Meaning Volatile liquids are liquids that transform easily. A measure of volatility is the vapor pressure. Volatility is essentially the tendency of a liquid to evaporate. A liquid's atmospheric pressure boiling point corresponds. It is a measure of the tendency of molecules and atoms to escape from a liquid or a solid. So a highly volatile liquid would show a great. Volatile Liquid Meaning.
From ar.inspiredpencil.com
Volatile Liquid Example Volatile Liquid Meaning Learn how volatility is measured by vapor pressure and boiling point, and how it affects. Volatile liquids are liquids that transform easily. A liquid's atmospheric pressure boiling point corresponds. Volatile substances are those that readily vaporize or sublimate in air. It is a measure of the tendency of molecules and atoms to escape from a liquid or a solid. Learn. Volatile Liquid Meaning.
From www.biologyonline.com
Volatile oil Definition and Examples Biology Online Dictionary Volatile Liquid Meaning A liquid that evaporates readily at normal temperature is known as a volatile liquid. Explore the concepts of evaporation, condensation, and dynamic equilibrium with. Learn how the vapor pressure of a liquid depends on temperature and intermolecular forces. It is a measure of the tendency of molecules and atoms to escape from a liquid or a solid. So a highly. Volatile Liquid Meaning.
From www.youtube.com
Diffusion of volatile liquid 29 5 2020 YouTube Volatile Liquid Meaning A liquid's atmospheric pressure boiling point corresponds. Explore the concepts of evaporation, condensation, and dynamic equilibrium with. A measure of volatility is the vapor pressure. Learn how the vapor pressure of a liquid depends on temperature and intermolecular forces. A liquid that evaporates readily at normal temperature is known as a volatile liquid. Volatile substances are those that readily vaporize. Volatile Liquid Meaning.
From blogofchemistry.blogspot.com
Blog of Chemistry Volatile Liquid Meaning Volatile substances are those that readily vaporize or sublimate in air. Volatility is essentially the tendency of a liquid to evaporate. Explore the concepts of evaporation, condensation, and dynamic equilibrium with. A liquid that evaporates readily at normal temperature is known as a volatile liquid. Learn how the vapor pressure of a liquid depends on temperature and intermolecular forces. Volatile. Volatile Liquid Meaning.
From studylib.net
03 The Molar Mass of a Volatile Liquid Volatile Liquid Meaning A volatile substance is one that vaporizes readily, meaning it has a high vapor pressure at a given temperature. Explore the concepts of evaporation, condensation, and dynamic equilibrium with. Learn how the vapor pressure of a liquid depends on temperature and intermolecular forces. Learn about the types, properties, and applications of volatile substances in chemistry. A measure of volatility is. Volatile Liquid Meaning.
From www.youtube.com
An ideal solution is formed by mixing two volatile liquids A and B. `X Volatile Liquid Meaning So a highly volatile liquid would show a great tendency to evaporate. Learn how the vapor pressure of a liquid depends on temperature and intermolecular forces. A volatile substance is one that vaporizes readily, meaning it has a high vapor pressure at a given temperature. Learn how volatility is measured by vapor pressure and boiling point, and how it affects.. Volatile Liquid Meaning.
From sciencenotes.org
Volatility Volatile Definition in Chemistry Volatile Liquid Meaning Volatile liquids are liquids that transform easily. Learn how the vapor pressure of a liquid depends on temperature and intermolecular forces. Learn how volatility is measured by vapor pressure and boiling point, and how it affects. It is a measure of the tendency of molecules and atoms to escape from a liquid or a solid. A liquid's atmospheric pressure boiling. Volatile Liquid Meaning.
From humananatomyall.blogspot.com
Volatile oil and its properties Human Anatomy Volatile Liquid Meaning A volatile substance is one that vaporizes readily, meaning it has a high vapor pressure at a given temperature. Volatility is essentially the tendency of a liquid to evaporate. Volatile liquids are liquids that transform easily. A liquid that evaporates readily at normal temperature is known as a volatile liquid. It is a measure of the tendency of molecules and. Volatile Liquid Meaning.
From www.sliderbase.com
Vapor Pressure of Solutions Presentation Chemistry Volatile Liquid Meaning Volatile substances are those that readily vaporize or sublimate in air. Learn how the vapor pressure of a liquid depends on temperature and intermolecular forces. Volatility is essentially the tendency of a liquid to evaporate. A liquid that evaporates readily at normal temperature is known as a volatile liquid. Volatile liquids are liquids that transform easily. So a highly volatile. Volatile Liquid Meaning.
From www.slideserve.com
PPT Volatile Substances PowerPoint Presentation, free download ID Volatile Liquid Meaning Learn about the types, properties, and applications of volatile substances in chemistry. Learn how the vapor pressure of a liquid depends on temperature and intermolecular forces. Volatility is essentially the tendency of a liquid to evaporate. Explore the concepts of evaporation, condensation, and dynamic equilibrium with. A measure of volatility is the vapor pressure. Volatile substances are those that readily. Volatile Liquid Meaning.
From www.slideshare.net
Solutions Powerpoint Volatile Liquid Meaning Learn about the types, properties, and applications of volatile substances in chemistry. Volatile liquids are liquids that transform easily. Volatility is essentially the tendency of a liquid to evaporate. It is a measure of the tendency of molecules and atoms to escape from a liquid or a solid. A liquid that evaporates readily at normal temperature is known as a. Volatile Liquid Meaning.
From shaunmwilliams.com
Chapter 11 Presentation Volatile Liquid Meaning Learn how the vapor pressure of a liquid depends on temperature and intermolecular forces. A volatile substance is one that vaporizes readily, meaning it has a high vapor pressure at a given temperature. It is a measure of the tendency of molecules and atoms to escape from a liquid or a solid. A measure of volatility is the vapor pressure.. Volatile Liquid Meaning.
From brainly.in
what are volatile liquids ? Brainly.in Volatile Liquid Meaning Volatile liquids are liquids that transform easily. Volatile substances are those that readily vaporize or sublimate in air. Learn how volatility is measured by vapor pressure and boiling point, and how it affects. Learn how the vapor pressure of a liquid depends on temperature and intermolecular forces. A liquid's atmospheric pressure boiling point corresponds. A liquid that evaporates readily at. Volatile Liquid Meaning.
From studylib.net
Simple Distillation of a Volatile Liquid Volatile Liquid Meaning So a highly volatile liquid would show a great tendency to evaporate. Volatility is essentially the tendency of a liquid to evaporate. A volatile substance is one that vaporizes readily, meaning it has a high vapor pressure at a given temperature. A measure of volatility is the vapor pressure. Learn how volatility is measured by vapor pressure and boiling point,. Volatile Liquid Meaning.
From www.youtube.com
Molecular weight of volatile liquid (part 2) YouTube Volatile Liquid Meaning Volatile liquids are liquids that transform easily. A measure of volatility is the vapor pressure. A liquid that evaporates readily at normal temperature is known as a volatile liquid. Learn how the vapor pressure of a liquid depends on temperature and intermolecular forces. It is a measure of the tendency of molecules and atoms to escape from a liquid or. Volatile Liquid Meaning.
From www.slideserve.com
PPT 4.5 Physical Properties of Covalent Molecules PowerPoint Volatile Liquid Meaning A measure of volatility is the vapor pressure. A liquid that evaporates readily at normal temperature is known as a volatile liquid. Explore the concepts of evaporation, condensation, and dynamic equilibrium with. So a highly volatile liquid would show a great tendency to evaporate. It is a measure of the tendency of molecules and atoms to escape from a liquid. Volatile Liquid Meaning.
From www.slideshare.net
Chapter 12.3 Changes of State Volatile Liquid Meaning A measure of volatility is the vapor pressure. A volatile substance is one that vaporizes readily, meaning it has a high vapor pressure at a given temperature. It is a measure of the tendency of molecules and atoms to escape from a liquid or a solid. Volatile substances are those that readily vaporize or sublimate in air. Learn how volatility. Volatile Liquid Meaning.
From dreamcivil.com
Volatile Liquids Civil Engineering Dictionary Volatile Liquid Meaning Learn how volatility is measured by vapor pressure and boiling point, and how it affects. A liquid's atmospheric pressure boiling point corresponds. Volatile substances are those that readily vaporize or sublimate in air. Learn how the vapor pressure of a liquid depends on temperature and intermolecular forces. It is a measure of the tendency of molecules and atoms to escape. Volatile Liquid Meaning.
From questions-in.kunduz.com
Two volatile liquids A & B are mixed to f... Physical Chemistry Volatile Liquid Meaning Volatile substances are those that readily vaporize or sublimate in air. Volatility is essentially the tendency of a liquid to evaporate. A measure of volatility is the vapor pressure. A volatile substance is one that vaporizes readily, meaning it has a high vapor pressure at a given temperature. So a highly volatile liquid would show a great tendency to evaporate.. Volatile Liquid Meaning.
From slideplayer.com
Chapter 10 States of Matter & Water Cycle ppt download Volatile Liquid Meaning A liquid's atmospheric pressure boiling point corresponds. So a highly volatile liquid would show a great tendency to evaporate. A liquid that evaporates readily at normal temperature is known as a volatile liquid. It is a measure of the tendency of molecules and atoms to escape from a liquid or a solid. A volatile substance is one that vaporizes readily,. Volatile Liquid Meaning.
From www.slideserve.com
PPT Chapter 10 Liquids and Solids PowerPoint Presentation ID5888387 Volatile Liquid Meaning Learn about the types, properties, and applications of volatile substances in chemistry. A volatile substance is one that vaporizes readily, meaning it has a high vapor pressure at a given temperature. A liquid that evaporates readily at normal temperature is known as a volatile liquid. Explore the concepts of evaporation, condensation, and dynamic equilibrium with. It is a measure of. Volatile Liquid Meaning.
From www.youtube.com
V.P of a Solute is a Volatile liquid Part17 Unit2 Cbse chemistry Volatile Liquid Meaning Learn about the types, properties, and applications of volatile substances in chemistry. A volatile substance is one that vaporizes readily, meaning it has a high vapor pressure at a given temperature. Volatile substances are those that readily vaporize or sublimate in air. So a highly volatile liquid would show a great tendency to evaporate. Volatile liquids are liquids that transform. Volatile Liquid Meaning.
From pediaa.com
Difference Between Volatile and Nonvolatile Substances Definition Volatile Liquid Meaning Learn how the vapor pressure of a liquid depends on temperature and intermolecular forces. Explore the concepts of evaporation, condensation, and dynamic equilibrium with. Learn about the types, properties, and applications of volatile substances in chemistry. A measure of volatility is the vapor pressure. Volatility is essentially the tendency of a liquid to evaporate. So a highly volatile liquid would. Volatile Liquid Meaning.
From www.polytechnichub.com
Volatile matter Polytechnic Hub Volatile Liquid Meaning A volatile substance is one that vaporizes readily, meaning it has a high vapor pressure at a given temperature. Volatile liquids are liquids that transform easily. So a highly volatile liquid would show a great tendency to evaporate. It is a measure of the tendency of molecules and atoms to escape from a liquid or a solid. Learn how the. Volatile Liquid Meaning.