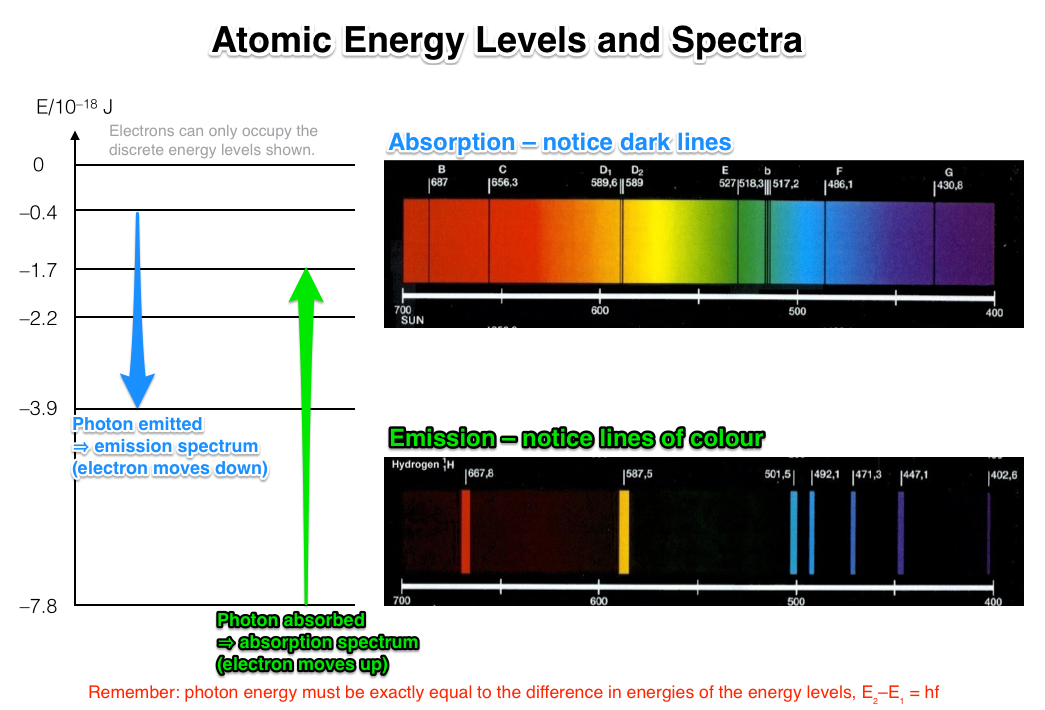
Flame Test Lab Atomic Emission And Electron Energy Levels . The energy absorbed could be in the form. Well, when an atom (or ion) absorbs energy, its electrons can make transitions from lower energy levels to higher energy levels. We can explain the emission of the. Flame tests are used to identify the presence of a. The objectives of this lab are to: The right amount of energy, an electron can move, or undergo a transition, from one energy level to the next. By gaining the right amount of energy, an electron can move, or undergo a transition, from. Electrons are said to be in the ground state under stable conditions. How does an electron move between energy levels? Electrons can be excited from energy in all forms (heat, light, chemical,. In this lab, you will perform flame tests for several metallic elements. When electrons are given energy from sources. This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Perform flame tests of metal cations in order to observe their characteristic colors, perform.
from electricaltestmodules.blogspot.com
Electrons can be excited from energy in all forms (heat, light, chemical,. We can explain the emission of the. The objectives of this lab are to: The right amount of energy, an electron can move, or undergo a transition, from one energy level to the next. In this lab, you will perform flame tests for several metallic elements. Well, when an atom (or ion) absorbs energy, its electrons can make transitions from lower energy levels to higher energy levels. How does an electron move between energy levels? The energy absorbed could be in the form. Electrons are said to be in the ground state under stable conditions. When electrons are given energy from sources.
Electrical test modules Flame tests and emission spectra
Flame Test Lab Atomic Emission And Electron Energy Levels When electrons are given energy from sources. Perform flame tests of metal cations in order to observe their characteristic colors, perform. The objectives of this lab are to: In this lab, you will perform flame tests for several metallic elements. Well, when an atom (or ion) absorbs energy, its electrons can make transitions from lower energy levels to higher energy levels. Electrons are said to be in the ground state under stable conditions. This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Flame tests are used to identify the presence of a. Electrons can be excited from energy in all forms (heat, light, chemical,. The right amount of energy, an electron can move, or undergo a transition, from one energy level to the next. By gaining the right amount of energy, an electron can move, or undergo a transition, from. The energy absorbed could be in the form. How does an electron move between energy levels? We can explain the emission of the. When electrons are given energy from sources.
From slidetodoc.com
Flame Test Electron Configuration Warm Up What is Flame Test Lab Atomic Emission And Electron Energy Levels Perform flame tests of metal cations in order to observe their characteristic colors, perform. This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. The objectives of this lab are to: We can explain the emission of the. By gaining the right amount of energy, an electron. Flame Test Lab Atomic Emission And Electron Energy Levels.
From studylib.net
Flame Test Lab Flame Test Lab Atomic Emission And Electron Energy Levels This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. The objectives of this lab are to: When electrons are given energy from sources. The energy absorbed could be in the form. Well, when an atom (or ion) absorbs energy, its electrons can make transitions from lower. Flame Test Lab Atomic Emission And Electron Energy Levels.
From studylib.net
Emission Spectra and Flame Tests Flame Test Lab Atomic Emission And Electron Energy Levels Well, when an atom (or ion) absorbs energy, its electrons can make transitions from lower energy levels to higher energy levels. When electrons are given energy from sources. How does an electron move between energy levels? This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Perform. Flame Test Lab Atomic Emission And Electron Energy Levels.
From ar.inspiredpencil.com
Atomic Spectrum Lab Setup Flame Test Lab Atomic Emission And Electron Energy Levels This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. When electrons are given energy from sources. The right amount of energy, an electron can move, or undergo a transition, from one energy level to the next. The energy absorbed could be in the form. We can. Flame Test Lab Atomic Emission And Electron Energy Levels.
From slideplayer.com
Electron Configuration ppt download Flame Test Lab Atomic Emission And Electron Energy Levels By gaining the right amount of energy, an electron can move, or undergo a transition, from. Perform flame tests of metal cations in order to observe their characteristic colors, perform. Flame tests are used to identify the presence of a. We can explain the emission of the. The objectives of this lab are to: When electrons are given energy from. Flame Test Lab Atomic Emission And Electron Energy Levels.
From studylib.net
FLAME TEST LAB Flame Test Lab Atomic Emission And Electron Energy Levels Well, when an atom (or ion) absorbs energy, its electrons can make transitions from lower energy levels to higher energy levels. The objectives of this lab are to: In this lab, you will perform flame tests for several metallic elements. Perform flame tests of metal cations in order to observe their characteristic colors, perform. The right amount of energy, an. Flame Test Lab Atomic Emission And Electron Energy Levels.
From www.markedbyteachers.com
Flame Tests GCSE Maths Marked by Flame Test Lab Atomic Emission And Electron Energy Levels The right amount of energy, an electron can move, or undergo a transition, from one energy level to the next. The energy absorbed could be in the form. By gaining the right amount of energy, an electron can move, or undergo a transition, from. This page describes how to perform a flame test for a range of metal ions, and. Flame Test Lab Atomic Emission And Electron Energy Levels.
From www.chegg.com
Solved Lab 11 Flame Tests and Atomic Emission Spectra Flame Test Lab Atomic Emission And Electron Energy Levels The right amount of energy, an electron can move, or undergo a transition, from one energy level to the next. When electrons are given energy from sources. Electrons can be excited from energy in all forms (heat, light, chemical,. Electrons are said to be in the ground state under stable conditions. Flame tests are used to identify the presence of. Flame Test Lab Atomic Emission And Electron Energy Levels.
From athensmutualaid.net
Atomic Emission And Flame Test Lab Answers › Athens Mutual Student Corner Flame Test Lab Atomic Emission And Electron Energy Levels Electrons are said to be in the ground state under stable conditions. We can explain the emission of the. How does an electron move between energy levels? Perform flame tests of metal cations in order to observe their characteristic colors, perform. Well, when an atom (or ion) absorbs energy, its electrons can make transitions from lower energy levels to higher. Flame Test Lab Atomic Emission And Electron Energy Levels.
From www.flinnsci.com
Flame Tests Atomic Emission and Electron Energy Levels—ChemTopic™ Lab Flame Test Lab Atomic Emission And Electron Energy Levels The energy absorbed could be in the form. Flame tests are used to identify the presence of a. By gaining the right amount of energy, an electron can move, or undergo a transition, from. In this lab, you will perform flame tests for several metallic elements. Electrons can be excited from energy in all forms (heat, light, chemical,. This page. Flame Test Lab Atomic Emission And Electron Energy Levels.
From www.youtube.com
Flame photometry/Flame Emission Spectroscopy (FES)/Atomic emission Flame Test Lab Atomic Emission And Electron Energy Levels The right amount of energy, an electron can move, or undergo a transition, from one energy level to the next. Perform flame tests of metal cations in order to observe their characteristic colors, perform. The energy absorbed could be in the form. The objectives of this lab are to: How does an electron move between energy levels? Electrons can be. Flame Test Lab Atomic Emission And Electron Energy Levels.
From www.mrpalermo.com
Virtual Lab Flame Test & Spectroscopy Mr. Palermo's Flipped Flame Test Lab Atomic Emission And Electron Energy Levels This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. How does an electron move between energy levels? The objectives of this lab are to: The right amount of energy, an electron can move, or undergo a transition, from one energy level to the next. By gaining. Flame Test Lab Atomic Emission And Electron Energy Levels.
From www.slideserve.com
PPT The ELECTRON Wave Particle Duality PowerPoint Presentation Flame Test Lab Atomic Emission And Electron Energy Levels This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. We can explain the emission of the. Electrons can be excited from energy in all forms (heat, light, chemical,. Electrons are said to be in the ground state under stable conditions. Well, when an atom (or ion). Flame Test Lab Atomic Emission And Electron Energy Levels.
From www.slideserve.com
PPT Atomic Absorption and Emission PowerPoint Presentation, free Flame Test Lab Atomic Emission And Electron Energy Levels The objectives of this lab are to: When electrons are given energy from sources. Electrons are said to be in the ground state under stable conditions. Well, when an atom (or ion) absorbs energy, its electrons can make transitions from lower energy levels to higher energy levels. We can explain the emission of the. The energy absorbed could be in. Flame Test Lab Atomic Emission And Electron Energy Levels.
From www.studocu.com
Atomic Emission and Flame Test Lab completed (2) Investigation Manual Flame Test Lab Atomic Emission And Electron Energy Levels We can explain the emission of the. Electrons are said to be in the ground state under stable conditions. Perform flame tests of metal cations in order to observe their characteristic colors, perform. Electrons can be excited from energy in all forms (heat, light, chemical,. The right amount of energy, an electron can move, or undergo a transition, from one. Flame Test Lab Atomic Emission And Electron Energy Levels.
From www.sliderbase.com
Electrons are particles…right? Flame Test Lab Atomic Emission And Electron Energy Levels Well, when an atom (or ion) absorbs energy, its electrons can make transitions from lower energy levels to higher energy levels. Electrons can be excited from energy in all forms (heat, light, chemical,. Electrons are said to be in the ground state under stable conditions. Perform flame tests of metal cations in order to observe their characteristic colors, perform. This. Flame Test Lab Atomic Emission And Electron Energy Levels.
From athensmutualaid.net
Atomic Emission And Flame Test Lab Answers › Athens Mutual Student Corner Flame Test Lab Atomic Emission And Electron Energy Levels The energy absorbed could be in the form. The right amount of energy, an electron can move, or undergo a transition, from one energy level to the next. This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. By gaining the right amount of energy, an electron. Flame Test Lab Atomic Emission And Electron Energy Levels.
From www.youtube.com
Atomic Spectroscopy Flame Emission The Flame Test YouTube Flame Test Lab Atomic Emission And Electron Energy Levels Electrons can be excited from energy in all forms (heat, light, chemical,. Well, when an atom (or ion) absorbs energy, its electrons can make transitions from lower energy levels to higher energy levels. Electrons are said to be in the ground state under stable conditions. By gaining the right amount of energy, an electron can move, or undergo a transition,. Flame Test Lab Atomic Emission And Electron Energy Levels.
From www.chegg.com
Solved Lab \4Flame Test Data Table Part A Simple Flame Flame Test Lab Atomic Emission And Electron Energy Levels This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Electrons can be excited from energy in all forms (heat, light, chemical,. We can explain the emission of the. The energy absorbed could be in the form. Well, when an atom (or ion) absorbs energy, its electrons. Flame Test Lab Atomic Emission And Electron Energy Levels.
From www.coursehero.com
[Solved] Atomic emission and flame test lab can i please get help. Data Flame Test Lab Atomic Emission And Electron Energy Levels The objectives of this lab are to: Well, when an atom (or ion) absorbs energy, its electrons can make transitions from lower energy levels to higher energy levels. Electrons are said to be in the ground state under stable conditions. How does an electron move between energy levels? In this lab, you will perform flame tests for several metallic elements.. Flame Test Lab Atomic Emission And Electron Energy Levels.
From www.studocu.com
Atomic Emission and Flame Test Lab Questions Atomic Emission and Flame Test Lab Atomic Emission And Electron Energy Levels Well, when an atom (or ion) absorbs energy, its electrons can make transitions from lower energy levels to higher energy levels. When electrons are given energy from sources. Perform flame tests of metal cations in order to observe their characteristic colors, perform. The right amount of energy, an electron can move, or undergo a transition, from one energy level to. Flame Test Lab Atomic Emission And Electron Energy Levels.
From www.slideserve.com
PPT Atomic Absorption and Emission PowerPoint Presentation, free Flame Test Lab Atomic Emission And Electron Energy Levels When electrons are given energy from sources. This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. How does an electron move between energy levels? Electrons are said to be in the ground state under stable conditions. We can explain the emission of the. Flame tests are. Flame Test Lab Atomic Emission And Electron Energy Levels.
From www.slideserve.com
PPT Atomic Absorption and Emission PowerPoint Presentation, free Flame Test Lab Atomic Emission And Electron Energy Levels Flame tests are used to identify the presence of a. How does an electron move between energy levels? By gaining the right amount of energy, an electron can move, or undergo a transition, from. We can explain the emission of the. Electrons can be excited from energy in all forms (heat, light, chemical,. Electrons are said to be in the. Flame Test Lab Atomic Emission And Electron Energy Levels.
From studymind.co.uk
Flame Emission Spectroscopy (GCSE Chemistry) Study Mind Flame Test Lab Atomic Emission And Electron Energy Levels We can explain the emission of the. The right amount of energy, an electron can move, or undergo a transition, from one energy level to the next. Well, when an atom (or ion) absorbs energy, its electrons can make transitions from lower energy levels to higher energy levels. This page describes how to perform a flame test for a range. Flame Test Lab Atomic Emission And Electron Energy Levels.
From www.youtube.com
Flame Test Lab YouTube Flame Test Lab Atomic Emission And Electron Energy Levels When electrons are given energy from sources. By gaining the right amount of energy, an electron can move, or undergo a transition, from. This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. The energy absorbed could be in the form. Electrons are said to be in. Flame Test Lab Atomic Emission And Electron Energy Levels.
From sciencenotes.org
Flame Test Colors and Procedure (Chemistry) Flame Test Lab Atomic Emission And Electron Energy Levels By gaining the right amount of energy, an electron can move, or undergo a transition, from. The energy absorbed could be in the form. Flame tests are used to identify the presence of a. The right amount of energy, an electron can move, or undergo a transition, from one energy level to the next. The objectives of this lab are. Flame Test Lab Atomic Emission And Electron Energy Levels.
From electricaltestmodules.blogspot.com
Electrical test modules Flame tests and emission spectra Flame Test Lab Atomic Emission And Electron Energy Levels This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. We can explain the emission of the. Well, when an atom (or ion) absorbs energy, its electrons can make transitions from lower energy levels to higher energy levels. By gaining the right amount of energy, an electron. Flame Test Lab Atomic Emission And Electron Energy Levels.
From sciencelessonsthatrock.com
Chemistry Flame Test Lab Science Lessons That Rock Flame Test Lab Atomic Emission And Electron Energy Levels Perform flame tests of metal cations in order to observe their characteristic colors, perform. When electrons are given energy from sources. The energy absorbed could be in the form. By gaining the right amount of energy, an electron can move, or undergo a transition, from. Electrons can be excited from energy in all forms (heat, light, chemical,. Flame tests are. Flame Test Lab Atomic Emission And Electron Energy Levels.
From athensmutualaid.net
Atomic Emission And Flame Test Lab Answers › Athens Mutual Student Corner Flame Test Lab Atomic Emission And Electron Energy Levels By gaining the right amount of energy, an electron can move, or undergo a transition, from. The energy absorbed could be in the form. We can explain the emission of the. When electrons are given energy from sources. This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color. Flame Test Lab Atomic Emission And Electron Energy Levels.
From www.youtube.com
Bohr Model Flame Test Explanation.mp4 YouTube Flame Test Lab Atomic Emission And Electron Energy Levels The energy absorbed could be in the form. How does an electron move between energy levels? By gaining the right amount of energy, an electron can move, or undergo a transition, from. This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. The right amount of energy,. Flame Test Lab Atomic Emission And Electron Energy Levels.
From mavink.com
Flame Emission Spectroscopy Diagram Flame Test Lab Atomic Emission And Electron Energy Levels This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. The energy absorbed could be in the form. Electrons are said to be in the ground state under stable conditions. Flame tests are used to identify the presence of a. We can explain the emission of the.. Flame Test Lab Atomic Emission And Electron Energy Levels.
From studylib.net
Atomic Spectra and the Flame Test Flame Test Lab Atomic Emission And Electron Energy Levels Flame tests are used to identify the presence of a. The objectives of this lab are to: The right amount of energy, an electron can move, or undergo a transition, from one energy level to the next. Electrons are said to be in the ground state under stable conditions. In this lab, you will perform flame tests for several metallic. Flame Test Lab Atomic Emission And Electron Energy Levels.
From www.animalia-life.club
Flame Test Lab Results Flame Test Lab Atomic Emission And Electron Energy Levels The energy absorbed could be in the form. How does an electron move between energy levels? The right amount of energy, an electron can move, or undergo a transition, from one energy level to the next. Well, when an atom (or ion) absorbs energy, its electrons can make transitions from lower energy levels to higher energy levels. This page describes. Flame Test Lab Atomic Emission And Electron Energy Levels.
From chem.libretexts.org
5 Flame Tests and Atomic Spectra (Experiment) Chemistry LibreTexts Flame Test Lab Atomic Emission And Electron Energy Levels Perform flame tests of metal cations in order to observe their characteristic colors, perform. The right amount of energy, an electron can move, or undergo a transition, from one energy level to the next. Well, when an atom (or ion) absorbs energy, its electrons can make transitions from lower energy levels to higher energy levels. Flame tests are used to. Flame Test Lab Atomic Emission And Electron Energy Levels.
From slideplayer.com
Quantum Mechanical Model ppt download Flame Test Lab Atomic Emission And Electron Energy Levels In this lab, you will perform flame tests for several metallic elements. Electrons are said to be in the ground state under stable conditions. The objectives of this lab are to: By gaining the right amount of energy, an electron can move, or undergo a transition, from. We can explain the emission of the. How does an electron move between. Flame Test Lab Atomic Emission And Electron Energy Levels.