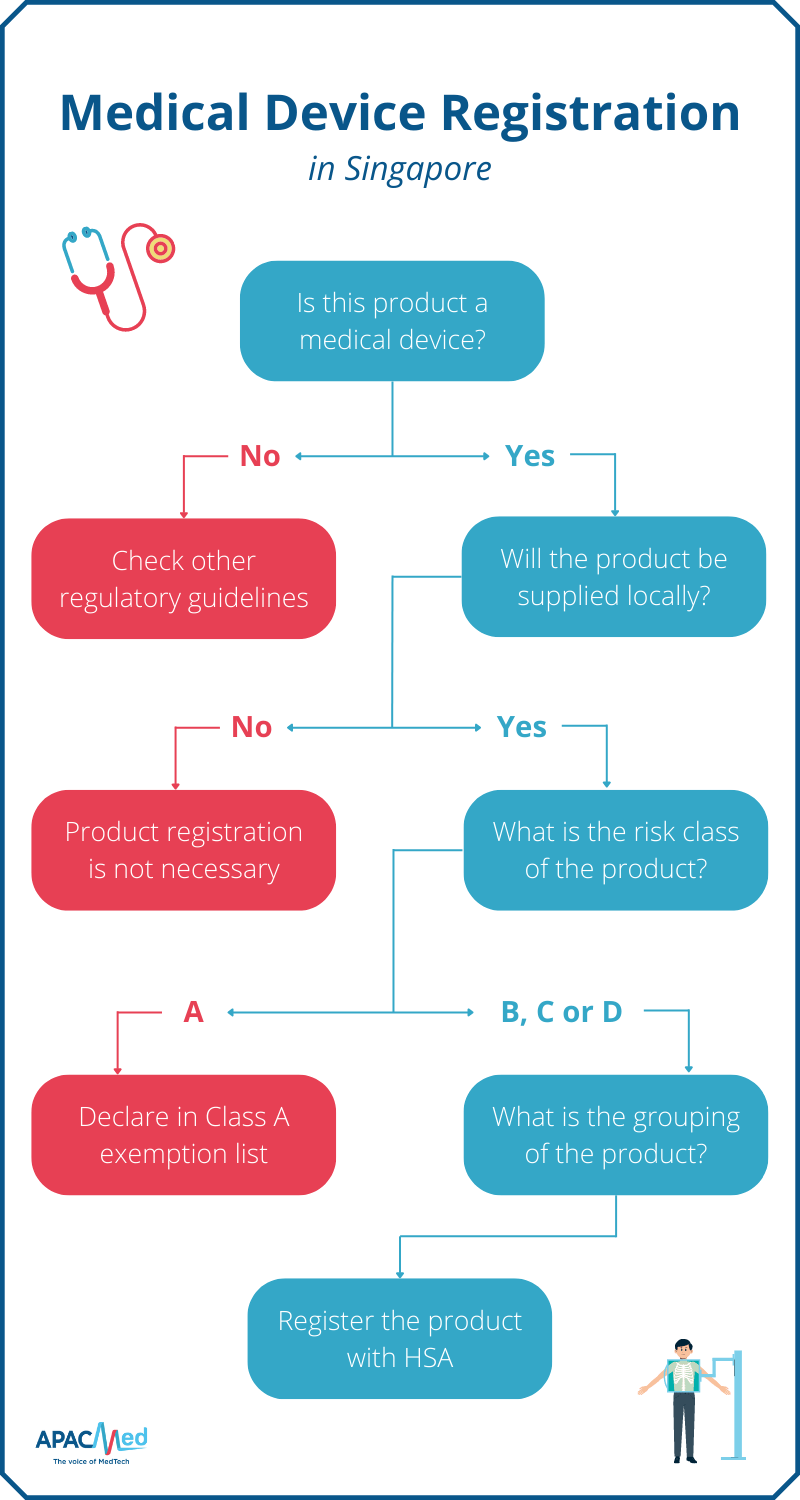
Medical Device Regulations Northern Ireland . The in vitro diagnostic medical device regulation (2017/746) has applied in northern ireland since 26 may 2022. These regulations make provision for the implementation in respect of northern ireland of regulation (eu) 2017/745 of the european. This instrument relates to the implementation of regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. Northern ireland continues to follow eu rules on medical devices and a ce marking is required for devices placed on the northern. Devices that have been ce marked in the eu can be sold in northern ireland. The draft medical devices (northern ireland protocol) regulations 2021 passed through the draft affirmative legislative procedure and were. The framework concerning medical devices in northern ireland is subject to the northern ireland protocol (the ni protocol) and. Manufacturers based outside of the uk wishing.
from apacmed.org
Manufacturers based outside of the uk wishing. These regulations make provision for the implementation in respect of northern ireland of regulation (eu) 2017/745 of the european. The framework concerning medical devices in northern ireland is subject to the northern ireland protocol (the ni protocol) and. The in vitro diagnostic medical device regulation (2017/746) has applied in northern ireland since 26 may 2022. This instrument relates to the implementation of regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. Devices that have been ce marked in the eu can be sold in northern ireland. The draft medical devices (northern ireland protocol) regulations 2021 passed through the draft affirmative legislative procedure and were. Northern ireland continues to follow eu rules on medical devices and a ce marking is required for devices placed on the northern.
Medical Device Regulation Importance and Examples in APAC
Medical Device Regulations Northern Ireland Devices that have been ce marked in the eu can be sold in northern ireland. These regulations make provision for the implementation in respect of northern ireland of regulation (eu) 2017/745 of the european. Devices that have been ce marked in the eu can be sold in northern ireland. The in vitro diagnostic medical device regulation (2017/746) has applied in northern ireland since 26 may 2022. The draft medical devices (northern ireland protocol) regulations 2021 passed through the draft affirmative legislative procedure and were. Northern ireland continues to follow eu rules on medical devices and a ce marking is required for devices placed on the northern. Manufacturers based outside of the uk wishing. This instrument relates to the implementation of regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. The framework concerning medical devices in northern ireland is subject to the northern ireland protocol (the ni protocol) and.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Medical Device Regulations Northern Ireland This instrument relates to the implementation of regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. The draft medical devices (northern ireland protocol) regulations 2021 passed through the draft affirmative legislative procedure and were. Devices that have been ce marked in the eu can be sold in northern ireland. The framework concerning medical. Medical Device Regulations Northern Ireland.
From operonstrategist.com
Effective phases of Medical Device Development Medical Device Regulations Northern Ireland The framework concerning medical devices in northern ireland is subject to the northern ireland protocol (the ni protocol) and. This instrument relates to the implementation of regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. These regulations make provision for the implementation in respect of northern ireland of regulation (eu) 2017/745 of the. Medical Device Regulations Northern Ireland.
From www.bmedicalsystems.com
FAQ on the European Medical Device Regulation B Medical Systems (US) Medical Device Regulations Northern Ireland Devices that have been ce marked in the eu can be sold in northern ireland. This instrument relates to the implementation of regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. These regulations make provision for the implementation in respect of northern ireland of regulation (eu) 2017/745 of the european. Manufacturers based outside. Medical Device Regulations Northern Ireland.
From www.eclevarmedtech.com
A Guide to Medical Devices Regulations Everything You Need to Know Medical Device Regulations Northern Ireland This instrument relates to the implementation of regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. Devices that have been ce marked in the eu can be sold in northern ireland. Northern ireland continues to follow eu rules on medical devices and a ce marking is required for devices placed on the northern.. Medical Device Regulations Northern Ireland.
From irishmedtechspringboard.ie
The Medical Technology Sector in Ireland Irish Medtech Springboard Medical Device Regulations Northern Ireland These regulations make provision for the implementation in respect of northern ireland of regulation (eu) 2017/745 of the european. This instrument relates to the implementation of regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. The framework concerning medical devices in northern ireland is subject to the northern ireland protocol (the ni protocol). Medical Device Regulations Northern Ireland.
From apacmed.org
Medical Device Regulation Importance and Examples in APAC Medical Device Regulations Northern Ireland Northern ireland continues to follow eu rules on medical devices and a ce marking is required for devices placed on the northern. Devices that have been ce marked in the eu can be sold in northern ireland. The in vitro diagnostic medical device regulation (2017/746) has applied in northern ireland since 26 may 2022. The draft medical devices (northern ireland. Medical Device Regulations Northern Ireland.
From www.apcerls.com
EU Medical Device Regulations APCER Life Sciences Medical Device Regulations Northern Ireland Devices that have been ce marked in the eu can be sold in northern ireland. The draft medical devices (northern ireland protocol) regulations 2021 passed through the draft affirmative legislative procedure and were. Northern ireland continues to follow eu rules on medical devices and a ce marking is required for devices placed on the northern. The framework concerning medical devices. Medical Device Regulations Northern Ireland.
From www.regdesk.co
MHRA Guidance on Registration of Medical Devices RegDesk Medical Device Regulations Northern Ireland This instrument relates to the implementation of regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. The in vitro diagnostic medical device regulation (2017/746) has applied in northern ireland since 26 may 2022. The draft medical devices (northern ireland protocol) regulations 2021 passed through the draft affirmative legislative procedure and were. Devices that. Medical Device Regulations Northern Ireland.
From www.orielstat.com
All Class 1 Medical Device Manufacturers Must Meet These Specific EU Medical Device Regulations Northern Ireland Manufacturers based outside of the uk wishing. Devices that have been ce marked in the eu can be sold in northern ireland. The in vitro diagnostic medical device regulation (2017/746) has applied in northern ireland since 26 may 2022. The framework concerning medical devices in northern ireland is subject to the northern ireland protocol (the ni protocol) and. This instrument. Medical Device Regulations Northern Ireland.
From www.eclevarmedtech.com
MDCG Comprehensive Guidance on Medical Devices Regulations in the EU Medical Device Regulations Northern Ireland Devices that have been ce marked in the eu can be sold in northern ireland. Manufacturers based outside of the uk wishing. The draft medical devices (northern ireland protocol) regulations 2021 passed through the draft affirmative legislative procedure and were. The in vitro diagnostic medical device regulation (2017/746) has applied in northern ireland since 26 may 2022. Northern ireland continues. Medical Device Regulations Northern Ireland.
From crfweb.com
Medical Device Regulations Medical Device Regulations Northern Ireland Devices that have been ce marked in the eu can be sold in northern ireland. Northern ireland continues to follow eu rules on medical devices and a ce marking is required for devices placed on the northern. These regulations make provision for the implementation in respect of northern ireland of regulation (eu) 2017/745 of the european. The in vitro diagnostic. Medical Device Regulations Northern Ireland.
From www.protoexpress.com
Medical Device Regulations for PCBA Sierra Circuits Medical Device Regulations Northern Ireland The in vitro diagnostic medical device regulation (2017/746) has applied in northern ireland since 26 may 2022. Northern ireland continues to follow eu rules on medical devices and a ce marking is required for devices placed on the northern. This instrument relates to the implementation of regulation (eu) 2017/745 of the european parliament and of the council of 5 april. Medical Device Regulations Northern Ireland.
From eurointervention.pcronline.com
Medical device regulation in Europe what is changing and how can I Medical Device Regulations Northern Ireland Northern ireland continues to follow eu rules on medical devices and a ce marking is required for devices placed on the northern. These regulations make provision for the implementation in respect of northern ireland of regulation (eu) 2017/745 of the european. The framework concerning medical devices in northern ireland is subject to the northern ireland protocol (the ni protocol) and.. Medical Device Regulations Northern Ireland.
From www.linkedin.com
Medical Device Regulatory Guide (MDRG) on LinkedIn Regulation of Medical Device Regulations Northern Ireland Manufacturers based outside of the uk wishing. These regulations make provision for the implementation in respect of northern ireland of regulation (eu) 2017/745 of the european. Northern ireland continues to follow eu rules on medical devices and a ce marking is required for devices placed on the northern. Devices that have been ce marked in the eu can be sold. Medical Device Regulations Northern Ireland.
From kpmg.com
EU Medical Devices Regulations MDR Safety KPMG Ireland Medical Device Regulations Northern Ireland This instrument relates to the implementation of regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. The in vitro diagnostic medical device regulation (2017/746) has applied in northern ireland since 26 may 2022. Devices that have been ce marked in the eu can be sold in northern ireland. These regulations make provision for. Medical Device Regulations Northern Ireland.
From readmagazine.com
Unlocking the Secrets of Medical Device Regulations A Comprehensive Medical Device Regulations Northern Ireland This instrument relates to the implementation of regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. The framework concerning medical devices in northern ireland is subject to the northern ireland protocol (the ni protocol) and. The in vitro diagnostic medical device regulation (2017/746) has applied in northern ireland since 26 may 2022. Northern. Medical Device Regulations Northern Ireland.
From operonstrategist.com
Complying with SaMD Expert Guidance on Regulations for Software as Medical Device Regulations Northern Ireland The draft medical devices (northern ireland protocol) regulations 2021 passed through the draft affirmative legislative procedure and were. Devices that have been ce marked in the eu can be sold in northern ireland. Northern ireland continues to follow eu rules on medical devices and a ce marking is required for devices placed on the northern. The in vitro diagnostic medical. Medical Device Regulations Northern Ireland.
From www.regdesk.co
MHRA Guidance on Registration of Medical Devices RegDesk Medical Device Regulations Northern Ireland Northern ireland continues to follow eu rules on medical devices and a ce marking is required for devices placed on the northern. These regulations make provision for the implementation in respect of northern ireland of regulation (eu) 2017/745 of the european. This instrument relates to the implementation of regulation (eu) 2017/745 of the european parliament and of the council of. Medical Device Regulations Northern Ireland.
From www.regdesk.co
MHRA Guidance on Clinical Investigations Northern Ireland RegDesk Medical Device Regulations Northern Ireland The framework concerning medical devices in northern ireland is subject to the northern ireland protocol (the ni protocol) and. This instrument relates to the implementation of regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. The in vitro diagnostic medical device regulation (2017/746) has applied in northern ireland since 26 may 2022. Devices. Medical Device Regulations Northern Ireland.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Regulations Northern Ireland The in vitro diagnostic medical device regulation (2017/746) has applied in northern ireland since 26 may 2022. Manufacturers based outside of the uk wishing. Devices that have been ce marked in the eu can be sold in northern ireland. The framework concerning medical devices in northern ireland is subject to the northern ireland protocol (the ni protocol) and. Northern ireland. Medical Device Regulations Northern Ireland.
From medicaldevices.freyrsolutions.com
UKCA Marking Requirements for Medical Devices Freyr Medical Devices Medical Device Regulations Northern Ireland This instrument relates to the implementation of regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. The draft medical devices (northern ireland protocol) regulations 2021 passed through the draft affirmative legislative procedure and were. These regulations make provision for the implementation in respect of northern ireland of regulation (eu) 2017/745 of the european.. Medical Device Regulations Northern Ireland.
From www.lexology.com
The MHRA's recent updates to the regulation of medical devices Lexology Medical Device Regulations Northern Ireland The in vitro diagnostic medical device regulation (2017/746) has applied in northern ireland since 26 may 2022. These regulations make provision for the implementation in respect of northern ireland of regulation (eu) 2017/745 of the european. Manufacturers based outside of the uk wishing. The draft medical devices (northern ireland protocol) regulations 2021 passed through the draft affirmative legislative procedure and. Medical Device Regulations Northern Ireland.
From www.ignitec.com
UK medical device regulations glossary What every medical... Medical Device Regulations Northern Ireland The framework concerning medical devices in northern ireland is subject to the northern ireland protocol (the ni protocol) and. Manufacturers based outside of the uk wishing. Northern ireland continues to follow eu rules on medical devices and a ce marking is required for devices placed on the northern. This instrument relates to the implementation of regulation (eu) 2017/745 of the. Medical Device Regulations Northern Ireland.
From gxp-training.com
Medical Device Regulation MDR 2017/745 Course and Certificate Medical Device Regulations Northern Ireland The in vitro diagnostic medical device regulation (2017/746) has applied in northern ireland since 26 may 2022. Northern ireland continues to follow eu rules on medical devices and a ce marking is required for devices placed on the northern. Devices that have been ce marked in the eu can be sold in northern ireland. These regulations make provision for the. Medical Device Regulations Northern Ireland.
From www.youtube.com
Understanding Medical Device Regulations YouTube Medical Device Regulations Northern Ireland The in vitro diagnostic medical device regulation (2017/746) has applied in northern ireland since 26 may 2022. These regulations make provision for the implementation in respect of northern ireland of regulation (eu) 2017/745 of the european. The draft medical devices (northern ireland protocol) regulations 2021 passed through the draft affirmative legislative procedure and were. Northern ireland continues to follow eu. Medical Device Regulations Northern Ireland.
From www.vchri.ca
Medical Device Regulations and Guidelines VCH Research Institute Medical Device Regulations Northern Ireland The in vitro diagnostic medical device regulation (2017/746) has applied in northern ireland since 26 may 2022. This instrument relates to the implementation of regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. Manufacturers based outside of the uk wishing. The framework concerning medical devices in northern ireland is subject to the northern. Medical Device Regulations Northern Ireland.
From security.cybellum.com
Intro to Medical Device Standards & Regulations Cybellum Medical Device Regulations Northern Ireland The in vitro diagnostic medical device regulation (2017/746) has applied in northern ireland since 26 may 2022. Manufacturers based outside of the uk wishing. The framework concerning medical devices in northern ireland is subject to the northern ireland protocol (the ni protocol) and. Devices that have been ce marked in the eu can be sold in northern ireland. The draft. Medical Device Regulations Northern Ireland.
From bmuconsultants.com
How to Get Irish Medical Council Registration Step By Step Guideline Medical Device Regulations Northern Ireland Northern ireland continues to follow eu rules on medical devices and a ce marking is required for devices placed on the northern. Manufacturers based outside of the uk wishing. Devices that have been ce marked in the eu can be sold in northern ireland. The in vitro diagnostic medical device regulation (2017/746) has applied in northern ireland since 26 may. Medical Device Regulations Northern Ireland.
From www.slideserve.com
PPT Global Medical Device Regulations PowerPoint Presentation, free Medical Device Regulations Northern Ireland The in vitro diagnostic medical device regulation (2017/746) has applied in northern ireland since 26 may 2022. These regulations make provision for the implementation in respect of northern ireland of regulation (eu) 2017/745 of the european. This instrument relates to the implementation of regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. Manufacturers. Medical Device Regulations Northern Ireland.
From www.tuvsud.cn
Infographic The Medical Device Regulation TÜV南德 Medical Device Regulations Northern Ireland Northern ireland continues to follow eu rules on medical devices and a ce marking is required for devices placed on the northern. Devices that have been ce marked in the eu can be sold in northern ireland. The framework concerning medical devices in northern ireland is subject to the northern ireland protocol (the ni protocol) and. The draft medical devices. Medical Device Regulations Northern Ireland.
From www.youtube.com
What Students Need To Know About Medical Device Regulations. YouTube Medical Device Regulations Northern Ireland The framework concerning medical devices in northern ireland is subject to the northern ireland protocol (the ni protocol) and. These regulations make provision for the implementation in respect of northern ireland of regulation (eu) 2017/745 of the european. This instrument relates to the implementation of regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017. Medical Device Regulations Northern Ireland.
From www.studypool.com
SOLUTION Medical device regulations global overview and guiding Medical Device Regulations Northern Ireland Manufacturers based outside of the uk wishing. Northern ireland continues to follow eu rules on medical devices and a ce marking is required for devices placed on the northern. This instrument relates to the implementation of regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. Devices that have been ce marked in the. Medical Device Regulations Northern Ireland.
From www.innofour.com
Comply with Medical Device Regulations InnoFour Medical Device Regulations Northern Ireland Northern ireland continues to follow eu rules on medical devices and a ce marking is required for devices placed on the northern. The in vitro diagnostic medical device regulation (2017/746) has applied in northern ireland since 26 may 2022. Manufacturers based outside of the uk wishing. The framework concerning medical devices in northern ireland is subject to the northern ireland. Medical Device Regulations Northern Ireland.
From www.ignitec.com
How does UK medical devices regulations differ from EU MDR Medical Device Regulations Northern Ireland The framework concerning medical devices in northern ireland is subject to the northern ireland protocol (the ni protocol) and. This instrument relates to the implementation of regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. Devices that have been ce marked in the eu can be sold in northern ireland. The draft medical. Medical Device Regulations Northern Ireland.
From www.researchsolutions.com
European Medical Device Regulation Guide to simplify compliance 2021 Medical Device Regulations Northern Ireland Manufacturers based outside of the uk wishing. These regulations make provision for the implementation in respect of northern ireland of regulation (eu) 2017/745 of the european. The draft medical devices (northern ireland protocol) regulations 2021 passed through the draft affirmative legislative procedure and were. The framework concerning medical devices in northern ireland is subject to the northern ireland protocol (the. Medical Device Regulations Northern Ireland.