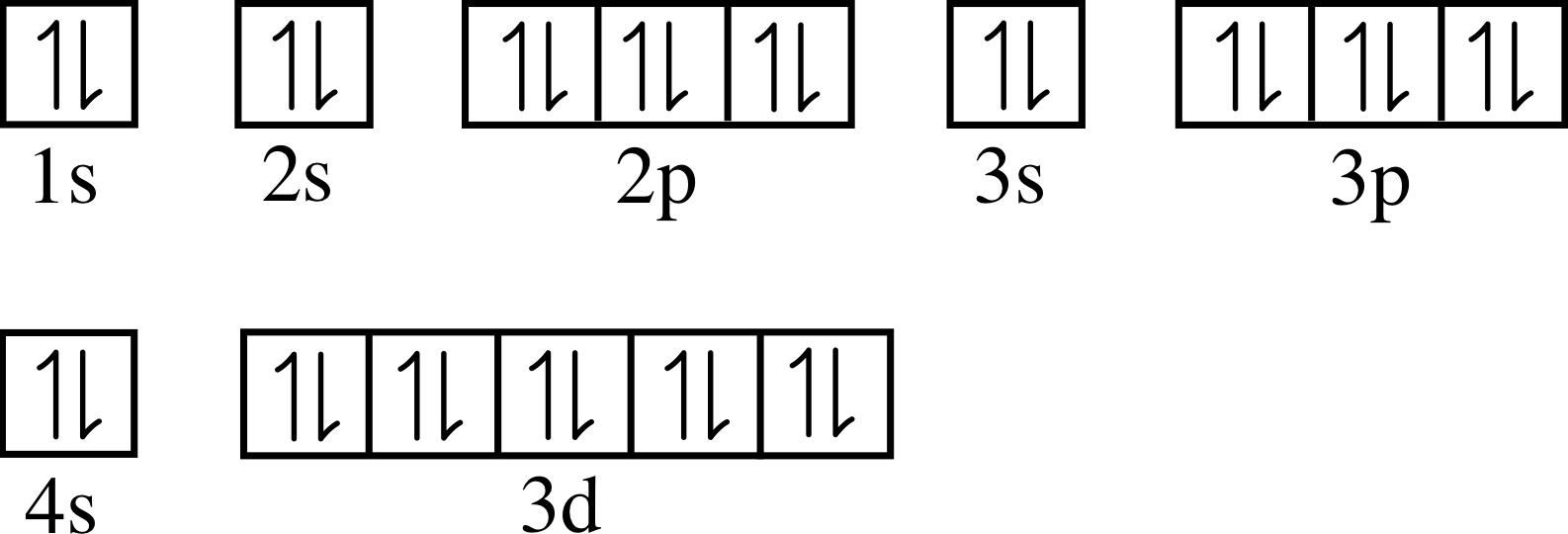
Zinc Electron Configuration Of Electrons . Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6. Electrons fill orbitals in increasing energy levels: Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. [ar] 3d10 4s2 is the electron configuration of zinc. Zinc has two valence electrons in its outer shell. Electron configuration of zinc is [ar] 3d10 4s2. Possible oxidation states are +2. The shorthand electron configuration (or noble gas. How many valence electrons does zinc have. Zinc’s electron configuration is 2,8,18,2 due to its 30 electrons. Electron configuration chart of all elements is mentioned in the table below. 2 in the first, 8 in the second, 18. It is located in group twelve, period four and block d. It is a moderately reactive metal and strong reducing agent.
from animalia-life.club
It is a moderately reactive metal and strong reducing agent. [ar] 3d10 4s2 is the electron configuration of zinc. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. The shorthand electron configuration (or noble gas. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. 2 in the first, 8 in the second, 18. Electron configuration of zinc is [ar] 3d10 4s2. Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6. Possible oxidation states are +2. Zinc’s electron configuration is 2,8,18,2 due to its 30 electrons.
Zinc Electron Configuration
Zinc Electron Configuration Of Electrons Electron configuration of zinc is [ar] 3d10 4s2. 2 in the first, 8 in the second, 18. Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6. It is located in group twelve, period four and block d. Zinc has two valence electrons in its outer shell. This element has 4 energy levels and in its outermost shell it has 2 electrons. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Possible oxidation states are +2. [ar] 3d10 4s2 is the electron configuration of zinc. Electrons fill orbitals in increasing energy levels: Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. Electron configuration of zinc is [ar] 3d10 4s2. How many valence electrons does zinc have. Electron configuration chart of all elements is mentioned in the table below. Zinc’s electron configuration is 2,8,18,2 due to its 30 electrons. The shorthand electron configuration (or noble gas.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Of Electrons It is a moderately reactive metal and strong reducing agent. Zinc’s electron configuration is 2,8,18,2 due to its 30 electrons. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas. It is located in. Zinc Electron Configuration Of Electrons.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Of Electrons Electron configuration chart of all elements is mentioned in the table below. Zinc has two valence electrons in its outer shell. The shorthand electron configuration (or noble gas. Possible oxidation states are +2. Electrons fill orbitals in increasing energy levels: It is a moderately reactive metal and strong reducing agent. It is located in group twelve, period four and block. Zinc Electron Configuration Of Electrons.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Of Electrons Electron configuration of zinc is [ar] 3d10 4s2. It is a moderately reactive metal and strong reducing agent. This element has 4 energy levels and in its outermost shell it has 2 electrons. It is located in group twelve, period four and block d. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration. Zinc Electron Configuration Of Electrons.
From material-properties.org
Zinc Protons Neutrons Electrons Electron Configuration Zinc Electron Configuration Of Electrons Zinc’s electron configuration is 2,8,18,2 due to its 30 electrons. It is a moderately reactive metal and strong reducing agent. This element has 4 energy levels and in its outermost shell it has 2 electrons. Electron configuration of zinc is [ar] 3d10 4s2. [ar] 3d10 4s2 is the electron configuration of zinc. Electron configuration chart of all elements is mentioned. Zinc Electron Configuration Of Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Zinc (Zn) Have? Zinc Electron Configuration Of Electrons This element has 4 energy levels and in its outermost shell it has 2 electrons. 2 in the first, 8 in the second, 18. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. Electrons fill orbitals in increasing energy levels: Electron configuration chart of all elements is. Zinc Electron Configuration Of Electrons.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements Zinc Electron Configuration Of Electrons Zinc’s electron configuration is 2,8,18,2 due to its 30 electrons. Electron configuration of zinc is [ar] 3d10 4s2. [ar] 3d10 4s2 is the electron configuration of zinc. Electrons fill orbitals in increasing energy levels: It is a moderately reactive metal and strong reducing agent. Zinc has two valence electrons in its outer shell. Its atomic number is 30, so its. Zinc Electron Configuration Of Electrons.
From www.sciencephoto.com
Zinc electron configuration Stock Image C029/5029 Science Photo Zinc Electron Configuration Of Electrons The shorthand electron configuration (or noble gas. This element has 4 energy levels and in its outermost shell it has 2 electrons. Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. It is located. Zinc Electron Configuration Of Electrons.
From www.youtube.com
Write electronic configuration of Zn & Zn2+ YouTube Zinc Electron Configuration Of Electrons Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6. Zinc has two valence electrons in its outer shell. How many valence electrons does zinc have. Electron configuration of zinc is [ar] 3d10 4s2. 2 in the first, 8 in the second, 18. It is a moderately reactive metal and strong reducing agent. Find the full electronic. Zinc Electron Configuration Of Electrons.
From www.youtube.com
Electron Configuration for Zn and Zn2+ (Zinc and Zinc ion) YouTube Zinc Electron Configuration Of Electrons Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Electrons fill orbitals in increasing energy levels: How many valence electrons does zinc have. Electron configuration of zinc is [ar] 3d10 4s2. Electron configuration chart of all elements is mentioned in the table below. Zinc’s electron configuration is 2,8,18,2 due to its 30. Zinc Electron Configuration Of Electrons.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Of Electrons It is located in group twelve, period four and block d. Electrons fill orbitals in increasing energy levels: Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6. Possible oxidation states are +2. How many valence electrons does zinc have. It is a moderately reactive metal and strong reducing agent. The shorthand electron configuration (or noble gas.. Zinc Electron Configuration Of Electrons.
From www.shutterstock.com
Zinc Zn Element Sphere Electron Shell Stock Vector (Royalty Free Zinc Electron Configuration Of Electrons Zinc has two valence electrons in its outer shell. How many valence electrons does zinc have. It is located in group twelve, period four and block d. Zinc’s electron configuration is 2,8,18,2 due to its 30 electrons. It is a moderately reactive metal and strong reducing agent. Possible oxidation states are +2. Electron configuration of zinc is [ar] 3d10 4s2.. Zinc Electron Configuration Of Electrons.
From www.pinterest.com
22 best Lessons to learn images on Pinterest School projects, Atomic Zinc Electron Configuration Of Electrons How many valence electrons does zinc have. Possible oxidation states are +2. It is a moderately reactive metal and strong reducing agent. This element has 4 energy levels and in its outermost shell it has 2 electrons. The shorthand electron configuration (or noble gas. [ar] 3d10 4s2 is the electron configuration of zinc. Electron configuration of zinc is [ar] 3d10. Zinc Electron Configuration Of Electrons.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Zinc Electron Configuration Of Electrons How many valence electrons does zinc have. Electrons fill orbitals in increasing energy levels: Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6. Zinc’s electron configuration is 2,8,18,2 due to its 30 electrons. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. [ar] 3d10 4s2 is the electron. Zinc Electron Configuration Of Electrons.
From ar.inspiredpencil.com
Zinc Electron Configuration Zinc Electron Configuration Of Electrons The shorthand electron configuration (or noble gas. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. Zinc has two valence electrons in its outer shell. Electron configuration of zinc is. Zinc Electron Configuration Of Electrons.
From www.freepik.com
Free Vector A zinc atom diagram Zinc Electron Configuration Of Electrons Electron configuration of zinc is [ar] 3d10 4s2. Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6. Zinc has two valence electrons in its outer shell. Possible oxidation states are +2. [ar] 3d10 4s2 is the electron configuration of zinc. Electron configuration chart of all elements is mentioned in the table below. How many valence electrons. Zinc Electron Configuration Of Electrons.
From www.teachoo.com
[Chemistry Class 12] Account for (c) Zinc is comparatively soft metal Zinc Electron Configuration Of Electrons Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Possible oxidation states are +2. The shorthand electron configuration (or noble gas. How many valence electrons does zinc have. Electron configuration. Zinc Electron Configuration Of Electrons.
From www.youtube.com
Electron Configuration of Zinc Zn Lesson YouTube Zinc Electron Configuration Of Electrons This element has 4 energy levels and in its outermost shell it has 2 electrons. How many valence electrons does zinc have. Electrons fill orbitals in increasing energy levels: Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. It is a moderately reactive metal and strong reducing agent. Zinc’s electron configuration is. Zinc Electron Configuration Of Electrons.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Of Electrons Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. [ar] 3d10 4s2 is the electron configuration of zinc. Zinc’s electron configuration is 2,8,18,2 due to its 30 electrons. It is located in group twelve, period four and block d. Electron configuration chart of all elements is mentioned. Zinc Electron Configuration Of Electrons.
From www.alamy.com
Symbol and electron diagram for Zinc illustration Stock Vector Image Zinc Electron Configuration Of Electrons Electrons fill orbitals in increasing energy levels: Zinc has two valence electrons in its outer shell. It is a moderately reactive metal and strong reducing agent. [ar] 3d10 4s2 is the electron configuration of zinc. It is located in group twelve, period four and block d. Electron configuration of zinc is [ar] 3d10 4s2. Possible oxidation states are +2. Find. Zinc Electron Configuration Of Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Zinc (Zn, Zn2+) Zinc Electron Configuration Of Electrons 2 in the first, 8 in the second, 18. Electrons fill orbitals in increasing energy levels: Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. It is a moderately reactive metal and strong reducing agent. Zinc’s electron configuration is 2,8,18,2 due to its 30 electrons. The shorthand. Zinc Electron Configuration Of Electrons.
From favpng.com
Zinc Atom Lewis Structure Bohr Model Electron Configuration, PNG Zinc Electron Configuration Of Electrons 2 in the first, 8 in the second, 18. Electrons fill orbitals in increasing energy levels: How many valence electrons does zinc have. This element has 4 energy levels and in its outermost shell it has 2 electrons. Zinc has two valence electrons in its outer shell. Zinc’s electron configuration is 2,8,18,2 due to its 30 electrons. The shorthand electron. Zinc Electron Configuration Of Electrons.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Of Electrons This element has 4 energy levels and in its outermost shell it has 2 electrons. Electrons fill orbitals in increasing energy levels: It is a moderately reactive metal and strong reducing agent. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Electron configuration of zinc is [ar] 3d10 4s2. The shorthand electron. Zinc Electron Configuration Of Electrons.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Of Electrons Possible oxidation states are +2. Electrons fill orbitals in increasing energy levels: The shorthand electron configuration (or noble gas. How many valence electrons does zinc have. [ar] 3d10 4s2 is the electron configuration of zinc. Electron configuration chart of all elements is mentioned in the table below. Find the full electronic configuration and valence electrons of any periodic element using. Zinc Electron Configuration Of Electrons.
From www.alamy.com
Zn Zinc, Periodic Table of the Elements, Shell Structure of Zinc Zinc Electron Configuration Of Electrons Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6. The shorthand electron configuration (or noble gas. Zinc has two valence electrons in its outer shell. How many valence electrons does zinc have. This element has 4 energy levels and in its outermost shell it has 2 electrons. Electron configuration chart of all elements is mentioned in. Zinc Electron Configuration Of Electrons.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Of Electrons Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6. The shorthand electron configuration (or noble gas. Possible oxidation states are +2. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. It is located in group twelve, period four and block d. How many. Zinc Electron Configuration Of Electrons.
From valenceelectrons.com
Complete Electron Configuration for Zinc (Zn, Zn2+ ion) Zinc Electron Configuration Of Electrons Zinc’s electron configuration is 2,8,18,2 due to its 30 electrons. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. This element has 4 energy levels and in its outermost shell it has 2 electrons. It is a moderately reactive metal and strong reducing agent. Zinc has an electron configuration of [ar]3d 10. Zinc Electron Configuration Of Electrons.
From material-properties.org
Zinc Protons Neutrons Electrons Electron Configuration Zinc Electron Configuration Of Electrons It is located in group twelve, period four and block d. 2 in the first, 8 in the second, 18. The shorthand electron configuration (or noble gas. [ar] 3d10 4s2 is the electron configuration of zinc. It is a moderately reactive metal and strong reducing agent. Electron configuration chart of all elements is mentioned in the table below. Zinc’s electron. Zinc Electron Configuration Of Electrons.
From elchoroukhost.net
Zinc Periodic Table Protons Elcho Table Zinc Electron Configuration Of Electrons Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6. Electrons fill orbitals in increasing energy levels: It is a moderately reactive metal and strong reducing agent. [ar] 3d10 4s2 is the electron configuration of zinc. The shorthand electron configuration (or noble gas. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member. Zinc Electron Configuration Of Electrons.
From www.britannica.com
zinc Properties, Uses, & Facts Britannica Zinc Electron Configuration Of Electrons Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. Electron configuration of zinc is [ar] 3d10 4s2. The shorthand electron configuration (or noble gas. Zinc’s electron configuration is 2,8,18,2 due to its 30 electrons. It is a moderately reactive metal and strong reducing agent. Possible oxidation states. Zinc Electron Configuration Of Electrons.
From periodictableguide.com
Zinc (Zn) Periodic Table (Element Information & More) Zinc Electron Configuration Of Electrons Electron configuration chart of all elements is mentioned in the table below. It is a moderately reactive metal and strong reducing agent. Zinc has two valence electrons in its outer shell. 2 in the first, 8 in the second, 18. Zinc’s electron configuration is 2,8,18,2 due to its 30 electrons. The shorthand electron configuration (or noble gas. It is located. Zinc Electron Configuration Of Electrons.
From manuallistcantabank.z21.web.core.windows.net
Zinc Orbital Diagram Zinc Electron Configuration Of Electrons 2 in the first, 8 in the second, 18. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. How many valence electrons does zinc have. It is a moderately reactive metal and strong reducing agent. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group. Zinc Electron Configuration Of Electrons.
From www.youtube.com
How to Find the Valence Electrons for Zinc (Zn) YouTube Zinc Electron Configuration Of Electrons 2 in the first, 8 in the second, 18. Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6. Electron configuration chart of all elements is mentioned in the table below. It is a moderately reactive metal and strong reducing agent. Electrons fill orbitals in increasing energy levels: [ar] 3d10 4s2 is the electron configuration of zinc.. Zinc Electron Configuration Of Electrons.
From www.youtube.com
zinc electronic configuration How to Write Zinc electronic Zinc Electron Configuration Of Electrons Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6. Possible oxidation states are +2. Electron configuration of zinc is [ar] 3d10 4s2. [ar] 3d10 4s2 is the electron configuration of zinc. Electrons fill orbitals in increasing energy levels: The shorthand electron configuration (or noble gas. Zinc’s electron configuration is 2,8,18,2 due to its 30 electrons. 2. Zinc Electron Configuration Of Electrons.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration Of Electrons This element has 4 energy levels and in its outermost shell it has 2 electrons. Electrons fill orbitals in increasing energy levels: It is located in group twelve, period four and block d. [ar] 3d10 4s2 is the electron configuration of zinc. Zinc’s electron configuration is 2,8,18,2 due to its 30 electrons. Electron configuration of zinc is [ar] 3d10 4s2.. Zinc Electron Configuration Of Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Zinc (Zn) Have? Zinc Electron Configuration Of Electrons Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Electron configuration of zinc is [ar] 3d10 4s2. The shorthand electron configuration (or noble gas. Possible oxidation states are +2. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. Zinc’s. Zinc Electron Configuration Of Electrons.