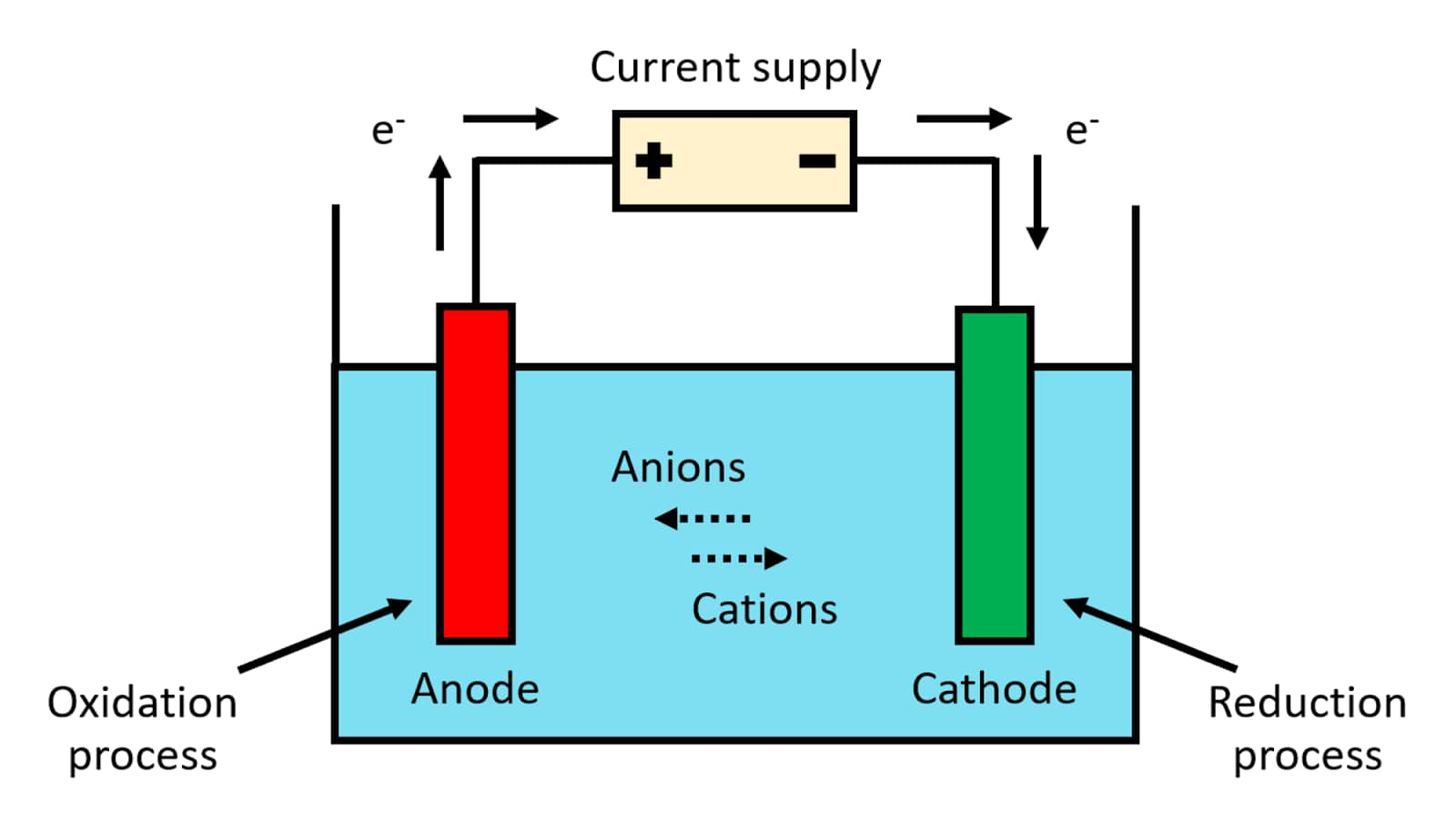
What Happens In Electrolytic Cell . electroplating and electrolytic refining of metals. the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous reaction (eo < 0) driven here by. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. it is possible to construct a cell that does work on a chemical system by driving an electric current through the system. components of electrochemical cells. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. what is an electrolytic cell? These cells are called electrolytic cells. Manufacture of hydrogen by electrolysis of water. Electrolysis is a fundamental part of.
from school.careers360.com
the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous reaction (eo < 0) driven here by. Electrolysis is a fundamental part of. what is an electrolytic cell? in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. These cells are called electrolytic cells. it is possible to construct a cell that does work on a chemical system by driving an electric current through the system. Manufacture of hydrogen by electrolysis of water. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. components of electrochemical cells. electroplating and electrolytic refining of metals.
Electrochemical Cell Overview, Structure, Properties & Uses
What Happens In Electrolytic Cell Manufacture of hydrogen by electrolysis of water. the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous reaction (eo < 0) driven here by. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. what is an electrolytic cell? Manufacture of hydrogen by electrolysis of water. Electrolysis is a fundamental part of. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. These cells are called electrolytic cells. electroplating and electrolytic refining of metals. components of electrochemical cells. it is possible to construct a cell that does work on a chemical system by driving an electric current through the system.
From www.slideserve.com
PPT CHAPTER 6 ELECTROLYSIS PowerPoint Presentation, free download What Happens In Electrolytic Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. it is possible to construct a cell that does work on a chemical system by driving an electric current through the system. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. These cells are. What Happens In Electrolytic Cell.
From saylordotorg.github.io
Electrolysis What Happens In Electrolytic Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. These cells are called electrolytic cells. Manufacture of hydrogen by electrolysis of water. what is an electrolytic cell? electroplating and electrolytic refining of metals. components of electrochemical cells. the electrolytic cell is used to perform electrolysis which is otherwise. What Happens In Electrolytic Cell.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki What Happens In Electrolytic Cell These cells are called electrolytic cells. what is an electrolytic cell? Electrolysis is a fundamental part of. the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous reaction (eo < 0) driven here by. it is possible to construct a cell that does work on a chemical system by driving an electric current through. What Happens In Electrolytic Cell.
From pandai.me
Electrolytic Cell What Happens In Electrolytic Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous reaction (eo < 0) driven here by. components of electrochemical cells. it is possible to construct a cell that does work on a chemical system by driving. What Happens In Electrolytic Cell.
From www.thoughtco.com
Electrochemical Cell Definition What Happens In Electrolytic Cell components of electrochemical cells. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. what is an electrolytic cell? electroplating and electrolytic refining of metals. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. Manufacture of hydrogen by electrolysis of water. . What Happens In Electrolytic Cell.
From spmscience.blog.onlinetuition.com.my
Electrolysis SPM Science What Happens In Electrolytic Cell the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous reaction (eo < 0) driven here by. electroplating and electrolytic refining of metals. These cells are called electrolytic cells. components of electrochemical cells. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. what is an. What Happens In Electrolytic Cell.
From www.slideserve.com
PPT The Electrolytic Cell PowerPoint Presentation, free download ID What Happens In Electrolytic Cell the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous reaction (eo < 0) driven here by. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. These cells are called electrolytic cells. Electrolysis is a fundamental part of. components of electrochemical cells. what is an electrolytic. What Happens In Electrolytic Cell.
From www.slideserve.com
PPT The Electrolytic Cell PowerPoint Presentation, free download ID What Happens In Electrolytic Cell components of electrochemical cells. Manufacture of hydrogen by electrolysis of water. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. electroplating and electrolytic refining of metals. These cells are called electrolytic cells. the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous reaction (eo < 0). What Happens In Electrolytic Cell.
From courses.lumenlearning.com
Electrolysis Chemistry What Happens In Electrolytic Cell in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. it is possible to construct a cell that does work on a chemical system by driving an electric current through the system. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. what is. What Happens In Electrolytic Cell.
From brilliant.org
Electrolytic Cells and Electrolysis Brilliant Math & Science Wiki What Happens In Electrolytic Cell in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. Electrolysis is a fundamental part of. components of electrochemical cells. it is possible to construct a cell that does work on a chemical system by driving an electric current through the system. These cells are called electrolytic cells. electroplating and. What Happens In Electrolytic Cell.
From www.pinterest.co.kr
What is Electrolytic cell Science chemistry, Electrochemical cell What Happens In Electrolytic Cell what is an electrolytic cell? An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. Manufacture of hydrogen by electrolysis of water. electroplating and electrolytic refining of metals. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. Electrolysis is a fundamental part of.. What Happens In Electrolytic Cell.
From www.researchgate.net
Schematic diagram of electrolytic cell Download Scientific Diagram What Happens In Electrolytic Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. components of electrochemical cells. These cells are called electrolytic cells. the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous reaction (eo < 0) driven here by. Electrolysis is a fundamental part of. what is an electrolytic. What Happens In Electrolytic Cell.
From mydigitalkemistry.com
What is Electrolytic Cell in Simple Words? What Happens In Electrolytic Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. what is an electrolytic cell? These cells are called electrolytic cells. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous. What Happens In Electrolytic Cell.
From www.edplace.com
Understand Electrolysis Worksheet EdPlace What Happens In Electrolytic Cell the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous reaction (eo < 0) driven here by. These cells are called electrolytic cells. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. electroplating and electrolytic refining of metals. components of electrochemical cells. it is possible. What Happens In Electrolytic Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5570772 What Happens In Electrolytic Cell it is possible to construct a cell that does work on a chemical system by driving an electric current through the system. components of electrochemical cells. These cells are called electrolytic cells. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. Manufacture of hydrogen by electrolysis of water. electroplating. What Happens In Electrolytic Cell.
From www.dreamstime.com
Electrolytic Cell Infographic Diagram with Components Stock Vector What Happens In Electrolytic Cell electroplating and electrolytic refining of metals. it is possible to construct a cell that does work on a chemical system by driving an electric current through the system. Manufacture of hydrogen by electrolysis of water. components of electrochemical cells. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. These. What Happens In Electrolytic Cell.
From www.researchgate.net
Diagram of electrolytic cell. Download Scientific Diagram What Happens In Electrolytic Cell Manufacture of hydrogen by electrolysis of water. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. These cells are called electrolytic cells. what is an electrolytic cell? components of electrochemical cells. Electrolysis is a fundamental part of. the electrolytic cell is used to perform electrolysis which is otherwise a. What Happens In Electrolytic Cell.
From circuitcoffeegirl89t1.z14.web.core.windows.net
What Forms At The Cathode During Electrolysis What Happens In Electrolytic Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. Electrolysis is a fundamental part of. it is possible to construct a cell that does work on a chemical system by driving an electric current through the system. the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous. What Happens In Electrolytic Cell.
From classnotes.org.in
Electrolytic Cells Chemistry, Class 12, Electro Chemistry What Happens In Electrolytic Cell components of electrochemical cells. the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous reaction (eo < 0) driven here by. Manufacture of hydrogen by electrolysis of water. Electrolysis is a fundamental part of. it is possible to construct a cell that does work on a chemical system by driving an electric current through. What Happens In Electrolytic Cell.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science What Happens In Electrolytic Cell what is an electrolytic cell? Electrolysis is a fundamental part of. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous reaction (eo < 0) driven here by. These cells are called electrolytic cells. Manufacture of hydrogen by. What Happens In Electrolytic Cell.
From chemistrypage.in
Electrolysis Definition, Principle and Electrolytic Cell What Happens In Electrolytic Cell it is possible to construct a cell that does work on a chemical system by driving an electric current through the system. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. what is. What Happens In Electrolytic Cell.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis What Happens In Electrolytic Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. Manufacture of hydrogen by electrolysis of water. the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous reaction (eo < 0) driven here by. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is. What Happens In Electrolytic Cell.
From byjus.com
An electrolytic cell is used to convert What Happens In Electrolytic Cell Manufacture of hydrogen by electrolysis of water. These cells are called electrolytic cells. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. electroplating and electrolytic refining of metals. it is possible to construct a cell that does work on a chemical system by driving an electric current through the system.. What Happens In Electrolytic Cell.
From youtube.com
9.5.3 Describe how current is conducted in an electrolytic cell IB What Happens In Electrolytic Cell These cells are called electrolytic cells. Electrolysis is a fundamental part of. electroplating and electrolytic refining of metals. what is an electrolytic cell? the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous reaction (eo < 0) driven here by. it is possible to construct a cell that does work on a chemical. What Happens In Electrolytic Cell.
From wisc.pb.unizin.org
M18Q8 Electrolysis Chem 103/104 Resource Book What Happens In Electrolytic Cell components of electrochemical cells. Manufacture of hydrogen by electrolysis of water. the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous reaction (eo < 0) driven here by. what is an electrolytic cell? These cells are called electrolytic cells. Electrolysis is a fundamental part of. An electrochemical cell splits the oxidant and reductant in. What Happens In Electrolytic Cell.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision What Happens In Electrolytic Cell what is an electrolytic cell? components of electrochemical cells. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous reaction. What Happens In Electrolytic Cell.
From wisc.pb.unizin.org
D41.4 Electrolysis Chemistry 109 Fall 2021 What Happens In Electrolytic Cell in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. electroplating and electrolytic refining of metals. components of electrochemical cells. Electrolysis is a fundamental part of. it is possible to construct a cell that does work on a chemical system by driving an electric current through the system. These cells. What Happens In Electrolytic Cell.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo What Happens In Electrolytic Cell Manufacture of hydrogen by electrolysis of water. components of electrochemical cells. Electrolysis is a fundamental part of. what is an electrolytic cell? the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous reaction (eo < 0) driven here by. These cells are called electrolytic cells. it is possible to construct a cell that. What Happens In Electrolytic Cell.
From general.chemistrysteps.com
Electrolysis Chemistry Steps What Happens In Electrolytic Cell These cells are called electrolytic cells. Electrolysis is a fundamental part of. components of electrochemical cells. what is an electrolytic cell? the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous reaction (eo < 0) driven here by. electroplating and electrolytic refining of metals. it is possible to construct a cell that. What Happens In Electrolytic Cell.
From www.coursehero.com
[Solved] Explain what happens in the electrolytic cell. AND Write What Happens In Electrolytic Cell what is an electrolytic cell? the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous reaction (eo < 0) driven here by. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. it is possible to construct a cell that does work on a chemical system by. What Happens In Electrolytic Cell.
From www.slideserve.com
PPT The Electrolytic Cell PowerPoint Presentation, free download ID What Happens In Electrolytic Cell Electrolysis is a fundamental part of. components of electrochemical cells. These cells are called electrolytic cells. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. it is possible to construct a cell that does work on a chemical system by driving an electric current through the system. in any. What Happens In Electrolytic Cell.
From slidetodoc.com
Electrolytic Cells Lab Lesson 10 Electrolytic cell Lab What Happens In Electrolytic Cell Electrolysis is a fundamental part of. These cells are called electrolytic cells. Manufacture of hydrogen by electrolysis of water. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous reaction (eo < 0) driven here by. in any. What Happens In Electrolytic Cell.
From school.careers360.com
Electrochemical Cell Overview, Structure, Properties & Uses What Happens In Electrolytic Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. Electrolysis is a fundamental part of. These cells are called electrolytic cells. what is an electrolytic cell? Manufacture of hydrogen by electrolysis of water. components of electrochemical cells. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction. What Happens In Electrolytic Cell.
From webmis.highland.cc.il.us
Electrolysis What Happens In Electrolytic Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. the electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous reaction (eo < 0) driven here by. electroplating and electrolytic refining. What Happens In Electrolytic Cell.
From general.chemistrysteps.com
Electrolysis Chemistry Steps What Happens In Electrolytic Cell electroplating and electrolytic refining of metals. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. it is possible to construct a cell that does work on a chemical system by driving an electric current through the system. components of electrochemical cells. Manufacture of hydrogen by electrolysis of water. Electrolysis. What Happens In Electrolytic Cell.