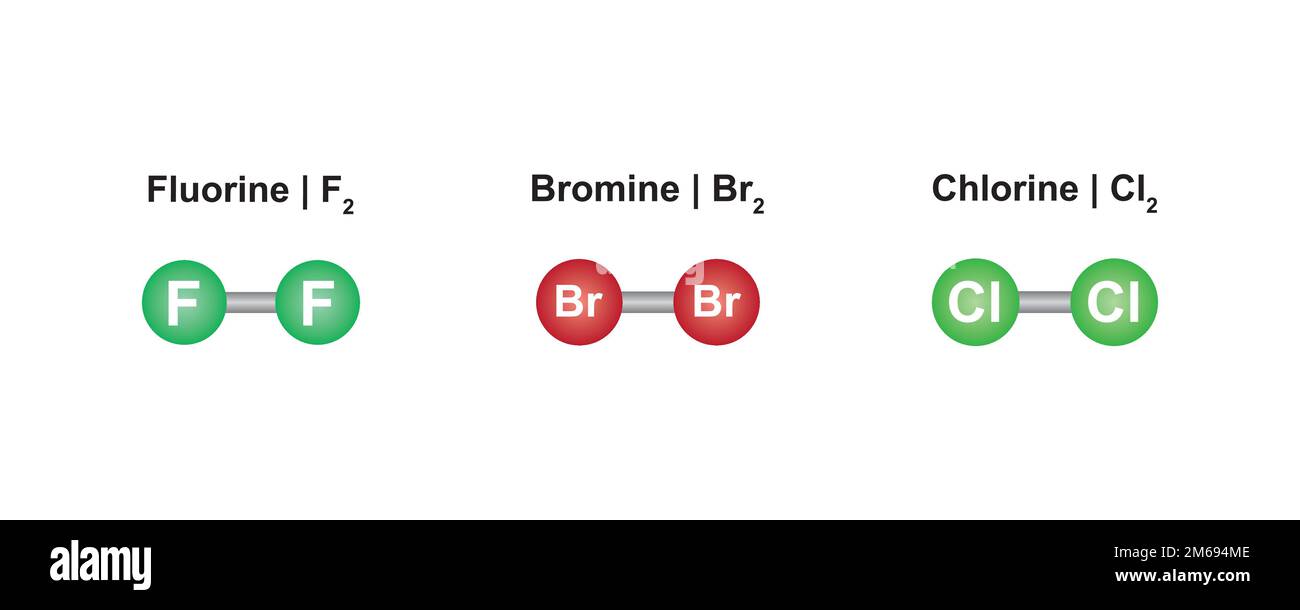
Fluorine Chlorine And Bromine . When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. The halogens become darker as you go down the group. Fluorine, chlorine, bromine and iodine. There are seven diatomic elements: The order of reactivity is chlorine > bromine > iodine. Halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i),. This page examines the trend in oxidizing ability of the group 17 elements (the halogens): These elements can exist in pure form in other arrangements. This is because chlorine could displace bromine and iodine, bromine could only. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine.
from www.alamy.com
These elements can exist in pure form in other arrangements. Fluorine, chlorine, bromine and iodine. This page examines the trend in oxidizing ability of the group 17 elements (the halogens): There are seven diatomic elements: The order of reactivity is chlorine > bromine > iodine. The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i),. This is because chlorine could displace bromine and iodine, bromine could only. The halogens become darker as you go down the group. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table.
Fluorine, Bromine and Chlorine Molecular Model of Atom. Vector
Fluorine Chlorine And Bromine Halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. This page examines the trend in oxidizing ability of the group 17 elements (the halogens): When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. These elements can exist in pure form in other arrangements. Halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. The order of reactivity is chlorine > bromine > iodine. There are seven diatomic elements: The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i),. This is because chlorine could displace bromine and iodine, bromine could only. The halogens become darker as you go down the group. Fluorine, chlorine, bromine and iodine. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine.
From www.researchgate.net
(PDF) Determination of Chlorine, Fluorine, Bromine, and Iodine in Coals Fluorine Chlorine And Bromine The halogens become darker as you go down the group. The order of reactivity is chlorine > bromine > iodine. These elements can exist in pure form in other arrangements. Halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. When chlorine (as a gas or dissolved in water) is added to sodium. Fluorine Chlorine And Bromine.
From www.youtube.com
How to draw Lewis dot structures Fluorine(F) Chlorine(Cl) Bromine(Br Fluorine Chlorine And Bromine When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. This page examines the trend in oxidizing ability of the group 17 elements (the halogens): The halogens become darker as you go down the group. These elements can exist in pure form in other arrangements. Fluorine, chlorine,. Fluorine Chlorine And Bromine.
From www.slideserve.com
PPT What do we know about fluorine, chlorine and bromine? PowerPoint Fluorine Chlorine And Bromine Fluorine, chlorine, bromine and iodine. The halogens become darker as you go down the group. This is because chlorine could displace bromine and iodine, bromine could only. There are seven diatomic elements: These elements can exist in pure form in other arrangements. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. Halogen, any of the six nonmetallic elements that constitute group. Fluorine Chlorine And Bromine.
From nap.nationalacademies.org
General References to the and Analytical Chemistry of the Fluorine Chlorine And Bromine There are seven diatomic elements: The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i),. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. This page examines the trend in oxidizing ability of the group 17 elements (the halogens): Fluorine, chlorine, bromine and iodine.. Fluorine Chlorine And Bromine.
From byjus.com
36. fluorine react with water release oxygen but chlorine bromine Fluorine Chlorine And Bromine Fluorine, chlorine, bromine and iodine. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i),. These elements can exist in pure form in other arrangements. There are seven diatomic elements: The halogens become darker as you go down the group. Halogen, any of the six nonmetallic elements that constitute group. Fluorine Chlorine And Bromine.
From stock.adobe.com
Diagram explaining Atomic Radius using diatomic molecules. Oxygen Fluorine Chlorine And Bromine This page examines the trend in oxidizing ability of the group 17 elements (the halogens): Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i),. The order of reactivity is chlorine > bromine > iodine. These elements can exist in pure form in other arrangements. The halogens become darker as. Fluorine Chlorine And Bromine.
From www.slideserve.com
PPT Winery Sanitation PowerPoint Presentation, free download ID5606303 Fluorine Chlorine And Bromine This page examines the trend in oxidizing ability of the group 17 elements (the halogens): These elements can exist in pure form in other arrangements. Halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. This is because chlorine could displace bromine and iodine, bromine. Fluorine Chlorine And Bromine.
From chemtest.com
Sulphur, Fluorine, Chlorine, Bromine and Iodine Eurofins Chemtest Limited Fluorine Chlorine And Bromine When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i),. The order of reactivity is chlorine > bromine > iodine. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. The halogens become darker as you go. Fluorine Chlorine And Bromine.
From www.alamy.com
Chlorine atomic structure Cut Out Stock Images & Pictures Alamy Fluorine Chlorine And Bromine This page examines the trend in oxidizing ability of the group 17 elements (the halogens): The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i),. This is because chlorine could displace bromine and iodine, bromine could only. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of. Fluorine Chlorine And Bromine.
From math.ucr.edu
Network Theory (Part 18) Fluorine Chlorine And Bromine Fluorine, chlorine, bromine and iodine. The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i),. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. The order of reactivity is chlorine > bromine > iodine. Halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. These elements can exist in pure form in. Fluorine Chlorine And Bromine.
From slideplayer.com
Yesterday’s Homework Questions p. 148 15 p. 150 13 p. 153 10, ppt Fluorine Chlorine And Bromine The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i),. Fluorine, chlorine, bromine and iodine. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. These elements can exist in pure form in other arrangements. The order of reactivity is chlorine > bromine > iodine. The halogens become darker as you go down the group. Halogen, any of the six. Fluorine Chlorine And Bromine.
From pediaa.com
Difference Between Bromine and Chlorine Fluorine Chlorine And Bromine The order of reactivity is chlorine > bromine > iodine. The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i),. These elements can exist in pure form in other arrangements. This page examines the trend in oxidizing ability of the group 17 elements (the halogens): The halogens become darker as you go down the group. Fluorine, chlorine, bromine. Fluorine Chlorine And Bromine.
From material-properties.org
Hydrogen and Fluorine Comparison Properties Material Properties Fluorine Chlorine And Bromine This is because chlorine could displace bromine and iodine, bromine could only. Halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. This page examines the trend in oxidizing ability of the group 17 elements (the halogens): When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the. Fluorine Chlorine And Bromine.
From chemisfast.blogspot.com
Halogen family elementspropertiesperiodic tableoxyacids Fluorine Chlorine And Bromine There are seven diatomic elements: When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. Halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. The order of reactivity is chlorine >. Fluorine Chlorine And Bromine.
From socratic.org
Why does fluorine have a higher ionization energy than bromine? Socratic Fluorine Chlorine And Bromine Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. There are seven diatomic elements: Fluorine, chlorine, bromine and iodine. The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i),. This page examines the trend in oxidizing. Fluorine Chlorine And Bromine.
From gcse-revision-chemistry.blogspot.com
Beccy's Chemistry Revision 2018 Section 2 c) Summary Fluorine Chlorine And Bromine There are seven diatomic elements: Fluorine, chlorine, bromine and iodine. This page examines the trend in oxidizing ability of the group 17 elements (the halogens): When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i),.. Fluorine Chlorine And Bromine.
From nap.nationalacademies.org
Table of Isotopes of Fluorine, Chlorine, Bromine and Iodine The Fluorine Chlorine And Bromine Halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. The halogens become darker as you go down the group. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. The order of reactivity is chlorine > bromine > iodine.. Fluorine Chlorine And Bromine.
From www.numerade.com
SOLVED Write the chemical formula for this molecule carbon hydrogen Fluorine Chlorine And Bromine The halogens become darker as you go down the group. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. This is because chlorine could displace bromine and iodine, bromine could only. This page examines the trend in oxidizing ability of the group 17 elements (the halogens):. Fluorine Chlorine And Bromine.
From nap.nationalacademies.org
Table of Isotopes of Fluorine, Chlorine, Bromine and Iodine The Fluorine Chlorine And Bromine The halogens become darker as you go down the group. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. There are seven diatomic elements: The order of reactivity is. Fluorine Chlorine And Bromine.
From bromineinformation.weebly.com
Bromine on the Periodic Table Fluorine Chlorine And Bromine The order of reactivity is chlorine > bromine > iodine. This page examines the trend in oxidizing ability of the group 17 elements (the halogens): Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. This is because chlorine could displace bromine and iodine, bromine could only. The halogens become darker as you go down the group. The halogen elements are fluorine. Fluorine Chlorine And Bromine.
From stock.adobe.com
Vecteur Stock Diatomic molecules diagram shows elements that exist as Fluorine Chlorine And Bromine This is because chlorine could displace bromine and iodine, bromine could only. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. The halogens become darker as you go down the group. These elements can exist in pure form in other arrangements. This page examines the trend in oxidizing ability of the group 17 elements (the halogens): The order of reactivity is. Fluorine Chlorine And Bromine.
From www.alamy.com
Fluorine, Bromine and Chlorine Molecular Model of Atom. Vector Fluorine Chlorine And Bromine The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i),. These elements can exist in pure form in other arrangements. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. The halogens become darker as you go down the group. The order of reactivity is. Fluorine Chlorine And Bromine.
From www.youtube.com
Fluorine *** YouTube Fluorine Chlorine And Bromine This is because chlorine could displace bromine and iodine, bromine could only. The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i),. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. There are seven diatomic elements: Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine.. Fluorine Chlorine And Bromine.
From www.researchgate.net
(a) Fluoride, (b) chlorine ( ) and chloride ( ), (c) total bromine, and Fluorine Chlorine And Bromine The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i),. The order of reactivity is chlorine > bromine > iodine. This is because chlorine could displace bromine and iodine, bromine could only. Fluorine, chlorine, bromine and iodine. This page examines the trend in oxidizing ability of the group 17 elements (the halogens): There are seven diatomic elements: These. Fluorine Chlorine And Bromine.
From www.researchgate.net
Recoveries of bromine (), chlorine (), fluorine (), iodine (), and Fluorine Chlorine And Bromine This page examines the trend in oxidizing ability of the group 17 elements (the halogens): The order of reactivity is chlorine > bromine > iodine. Fluorine, chlorine, bromine and iodine. Halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. There are seven diatomic elements: These elements can exist in pure form in. Fluorine Chlorine And Bromine.
From www.youtube.com
How to Balance F2 + Cl2 = ClF3 (Fluorine gas + Chlorine gas) YouTube Fluorine Chlorine And Bromine Fluorine, chlorine, bromine and iodine. This is because chlorine could displace bromine and iodine, bromine could only. Halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i),. The order of reactivity is chlorine > bromine > iodine. When chlorine (as a. Fluorine Chlorine And Bromine.
From peguys.com
Fluorine Chlorine Bromine Iodine Low Pressure Ampoules set in Periodic Fluorine Chlorine And Bromine Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i),. Halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine.. Fluorine Chlorine And Bromine.
From www.alamy.com
BrF3 bromine trifluoride CAS 7787715 chemical substance in white Fluorine Chlorine And Bromine The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i),. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. The halogens become darker as you go down the group. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. This is because chlorine could displace bromine. Fluorine Chlorine And Bromine.
From material-properties.org
Chlorine and Iodine Comparison Properties Material Properties Fluorine Chlorine And Bromine The order of reactivity is chlorine > bromine > iodine. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. This is because chlorine could displace bromine and iodine, bromine could only. Halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the. Fluorine Chlorine And Bromine.
From joelgordon.photoshelter.com
Chlorine Bromine Iodine Joel Gordon Photography Fluorine Chlorine And Bromine These elements can exist in pure form in other arrangements. This page examines the trend in oxidizing ability of the group 17 elements (the halogens): The halogens become darker as you go down the group. The order of reactivity is chlorine > bromine > iodine. There are seven diatomic elements: Halogen, any of the six nonmetallic elements that constitute group. Fluorine Chlorine And Bromine.
From www.alamy.com
halogens in gas jars Fl Cl Br I fluorine chlorine bromine iodine Stock Fluorine Chlorine And Bromine These elements can exist in pure form in other arrangements. There are seven diatomic elements: This is because chlorine could displace bromine and iodine, bromine could only. The order of reactivity is chlorine > bromine > iodine. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine.. Fluorine Chlorine And Bromine.
From www.vedantu.com
Going from fluorine, chlorine, bromine to iodine the electronegativity Fluorine Chlorine And Bromine Halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i),. Hydrogen, nitrogen, oxygen, fluorine, chlorine, iodine, and bromine. The halogens become darker as you go down the group. When chlorine (as a gas or dissolved in water) is added to sodium. Fluorine Chlorine And Bromine.
From amazon.com
The Halogen Elements Fluorine, Chlorine, Bromine, Iodine, Astatine Fluorine Chlorine And Bromine The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i),. This is because chlorine could displace bromine and iodine, bromine could only. The halogens become darker as you go down the group. There are seven diatomic elements: Fluorine, chlorine, bromine and iodine. This page examines the trend in oxidizing ability of the group 17 elements (the halogens): When. Fluorine Chlorine And Bromine.
From ecurrencythailand.com
Which Is The Least Reactive Fluorine Chlorine Bromine Iodine? 22 Most Fluorine Chlorine And Bromine These elements can exist in pure form in other arrangements. This is because chlorine could displace bromine and iodine, bromine could only. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. The halogens become darker as you go down the group. This page examines the trend. Fluorine Chlorine And Bromine.
From www.youtube.com
BrF Polar or Nonpolar (Bromine fluoride) YouTube Fluorine Chlorine And Bromine Fluorine, chlorine, bromine and iodine. The halogens become darker as you go down the group. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. The halogen elements are fluorine. Fluorine Chlorine And Bromine.