Chlorine Electronegative Than Fluorine . a) the fluorine atom is smaller than the chlorine atom, and there is less shielding from other shells of electrons. from what i remember, the trend is that the more right and up you go on the periodic table, the more. Electronegativity is used to predict whether a bond. and yes, chlorine has a higher electron affinity than fluorine. Chlorine is a bigger atom than fluorine. the higher electronegativity of fluorine as compared to chlorine (table \(\pageindex{1}\)), and the ability of chlorine to form more than one bond, means that higher fluorides of chlorine are also known, i.e., clf 3 and clf 5. Thus are less attracted to the nucleus, therefore. 1s 2 2s 2 2p x 2 2p y 2. why isn't chlorine as electronegative as fluorine? fluorine, though higher than chlorine in the periodic table, has a very small atomic size. That is because although fluorine wants to attract. chlorines electrons are better shielded and so feel less nuclear charge. 119 rows values for electronegativity run from 0 to 4.
from www.numerade.com
That is because although fluorine wants to attract. why isn't chlorine as electronegative as fluorine? and yes, chlorine has a higher electron affinity than fluorine. Thus are less attracted to the nucleus, therefore. chlorines electrons are better shielded and so feel less nuclear charge. a) the fluorine atom is smaller than the chlorine atom, and there is less shielding from other shells of electrons. 119 rows values for electronegativity run from 0 to 4. 1s 2 2s 2 2p x 2 2p y 2. Electronegativity is used to predict whether a bond. Chlorine is a bigger atom than fluorine.
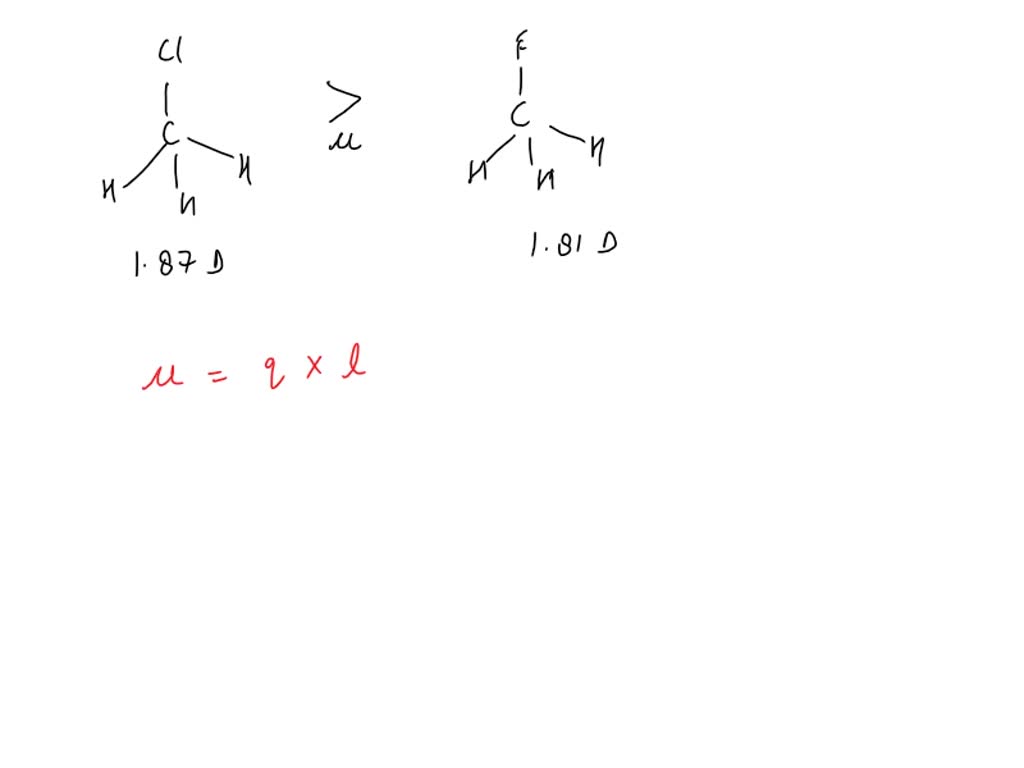
SOLVED Fluoromethane (CH3F, μ=1.81 D) has a smaller dipole moment
Chlorine Electronegative Than Fluorine Thus are less attracted to the nucleus, therefore. 1s 2 2s 2 2p x 2 2p y 2. Thus are less attracted to the nucleus, therefore. Electronegativity is used to predict whether a bond. chlorines electrons are better shielded and so feel less nuclear charge. fluorine, though higher than chlorine in the periodic table, has a very small atomic size. why isn't chlorine as electronegative as fluorine? a) the fluorine atom is smaller than the chlorine atom, and there is less shielding from other shells of electrons. and yes, chlorine has a higher electron affinity than fluorine. 119 rows values for electronegativity run from 0 to 4. the higher electronegativity of fluorine as compared to chlorine (table \(\pageindex{1}\)), and the ability of chlorine to form more than one bond, means that higher fluorides of chlorine are also known, i.e., clf 3 and clf 5. That is because although fluorine wants to attract. Chlorine is a bigger atom than fluorine. from what i remember, the trend is that the more right and up you go on the periodic table, the more.
From ecurrencythailand.com
Which Of The Following Has Highest Electron Affinity Of Fluorine Chlorine Electronegative Than Fluorine from what i remember, the trend is that the more right and up you go on the periodic table, the more. the higher electronegativity of fluorine as compared to chlorine (table \(\pageindex{1}\)), and the ability of chlorine to form more than one bond, means that higher fluorides of chlorine are also known, i.e., clf 3 and clf 5.. Chlorine Electronegative Than Fluorine.
From exoyzbldb.blob.core.windows.net
Is Chlorine Electronegative Or Electropositive at Otis King blog Chlorine Electronegative Than Fluorine a) the fluorine atom is smaller than the chlorine atom, and there is less shielding from other shells of electrons. fluorine, though higher than chlorine in the periodic table, has a very small atomic size. and yes, chlorine has a higher electron affinity than fluorine. chlorines electrons are better shielded and so feel less nuclear charge.. Chlorine Electronegative Than Fluorine.
From www.meritnation.com
pls explain why fluorine has less negative electron gain enthalpy than Chlorine Electronegative Than Fluorine the higher electronegativity of fluorine as compared to chlorine (table \(\pageindex{1}\)), and the ability of chlorine to form more than one bond, means that higher fluorides of chlorine are also known, i.e., clf 3 and clf 5. Thus are less attracted to the nucleus, therefore. and yes, chlorine has a higher electron affinity than fluorine. 119 rows. Chlorine Electronegative Than Fluorine.
From www.youtube.com
Why fluorine is most electronegative element 🔥 YouTube Chlorine Electronegative Than Fluorine Thus are less attracted to the nucleus, therefore. a) the fluorine atom is smaller than the chlorine atom, and there is less shielding from other shells of electrons. 119 rows values for electronegativity run from 0 to 4. why isn't chlorine as electronegative as fluorine? 1s 2 2s 2 2p x 2 2p y 2. Chlorine is. Chlorine Electronegative Than Fluorine.
From www.numerade.com
SOLVED Which element meets all of the following conditions? has all Chlorine Electronegative Than Fluorine 1s 2 2s 2 2p x 2 2p y 2. why isn't chlorine as electronegative as fluorine? Thus are less attracted to the nucleus, therefore. from what i remember, the trend is that the more right and up you go on the periodic table, the more. Electronegativity is used to predict whether a bond. a) the fluorine. Chlorine Electronegative Than Fluorine.
From byjus.com
why fluorine is more electronegative than oxygen. Chlorine Electronegative Than Fluorine a) the fluorine atom is smaller than the chlorine atom, and there is less shielding from other shells of electrons. Electronegativity is used to predict whether a bond. chlorines electrons are better shielded and so feel less nuclear charge. That is because although fluorine wants to attract. from what i remember, the trend is that the more. Chlorine Electronegative Than Fluorine.
From askfilo.com
If the electronegativities of fluorine and chlorine are 4.0 and 3.0 resp.. Chlorine Electronegative Than Fluorine from what i remember, the trend is that the more right and up you go on the periodic table, the more. Thus are less attracted to the nucleus, therefore. a) the fluorine atom is smaller than the chlorine atom, and there is less shielding from other shells of electrons. 119 rows values for electronegativity run from 0. Chlorine Electronegative Than Fluorine.
From www.youtube.com
Oxygen is more electronegative than chlorine. In the series of oxyacids Chlorine Electronegative Than Fluorine chlorines electrons are better shielded and so feel less nuclear charge. 119 rows values for electronegativity run from 0 to 4. Electronegativity is used to predict whether a bond. Thus are less attracted to the nucleus, therefore. why isn't chlorine as electronegative as fluorine? 1s 2 2s 2 2p x 2 2p y 2. from what. Chlorine Electronegative Than Fluorine.
From www.learnatnoon.com
Which Halogen Has The Lowest Electronegativity? Noon Academy Chlorine Electronegative Than Fluorine Chlorine is a bigger atom than fluorine. and yes, chlorine has a higher electron affinity than fluorine. 119 rows values for electronegativity run from 0 to 4. Thus are less attracted to the nucleus, therefore. 1s 2 2s 2 2p x 2 2p y 2. Electronegativity is used to predict whether a bond. why isn't chlorine as. Chlorine Electronegative Than Fluorine.
From www.numerade.com
SOLVED Fluoromethane (CH3F, μ=1.81 D) has a smaller dipole moment Chlorine Electronegative Than Fluorine a) the fluorine atom is smaller than the chlorine atom, and there is less shielding from other shells of electrons. fluorine, though higher than chlorine in the periodic table, has a very small atomic size. 119 rows values for electronegativity run from 0 to 4. Electronegativity is used to predict whether a bond. why isn't chlorine. Chlorine Electronegative Than Fluorine.
From www.nuclear-power.com
Fluorine Electron Affinity Electronegativity Ionization Energy of Chlorine Electronegative Than Fluorine 119 rows values for electronegativity run from 0 to 4. Electronegativity is used to predict whether a bond. 1s 2 2s 2 2p x 2 2p y 2. and yes, chlorine has a higher electron affinity than fluorine. from what i remember, the trend is that the more right and up you go on the periodic table,. Chlorine Electronegative Than Fluorine.
From exoyzbldb.blob.core.windows.net
Is Chlorine Electronegative Or Electropositive at Otis King blog Chlorine Electronegative Than Fluorine a) the fluorine atom is smaller than the chlorine atom, and there is less shielding from other shells of electrons. Electronegativity is used to predict whether a bond. why isn't chlorine as electronegative as fluorine? Thus are less attracted to the nucleus, therefore. 119 rows values for electronegativity run from 0 to 4. the higher electronegativity. Chlorine Electronegative Than Fluorine.
From dxorxejky.blob.core.windows.net
Does Chlorine Have A Larger Atomic Radius Than Fluorine at Leon Cain blog Chlorine Electronegative Than Fluorine a) the fluorine atom is smaller than the chlorine atom, and there is less shielding from other shells of electrons. chlorines electrons are better shielded and so feel less nuclear charge. from what i remember, the trend is that the more right and up you go on the periodic table, the more. Chlorine is a bigger atom. Chlorine Electronegative Than Fluorine.
From barkmanoil.com
Why Is Chlorine More Reactive Than Bromine? The 8 Top Answers Chlorine Electronegative Than Fluorine Chlorine is a bigger atom than fluorine. That is because although fluorine wants to attract. 119 rows values for electronegativity run from 0 to 4. chlorines electrons are better shielded and so feel less nuclear charge. the higher electronegativity of fluorine as compared to chlorine (table \(\pageindex{1}\)), and the ability of chlorine to form more than one. Chlorine Electronegative Than Fluorine.
From dxorxejky.blob.core.windows.net
Does Chlorine Have A Larger Atomic Radius Than Fluorine at Leon Cain blog Chlorine Electronegative Than Fluorine the higher electronegativity of fluorine as compared to chlorine (table \(\pageindex{1}\)), and the ability of chlorine to form more than one bond, means that higher fluorides of chlorine are also known, i.e., clf 3 and clf 5. That is because although fluorine wants to attract. a) the fluorine atom is smaller than the chlorine atom, and there is. Chlorine Electronegative Than Fluorine.
From www.numerade.com
SOLVED Oxygen is more electronegative than nitrogen; fluorine is more Chlorine Electronegative Than Fluorine why isn't chlorine as electronegative as fluorine? Chlorine is a bigger atom than fluorine. Electronegativity is used to predict whether a bond. chlorines electrons are better shielded and so feel less nuclear charge. and yes, chlorine has a higher electron affinity than fluorine. 1s 2 2s 2 2p x 2 2p y 2. the higher electronegativity. Chlorine Electronegative Than Fluorine.
From www.doubtnut.com
Fluorine is more electronegative than chlorine but pfluorobenzoic aci Chlorine Electronegative Than Fluorine 1s 2 2s 2 2p x 2 2p y 2. the higher electronegativity of fluorine as compared to chlorine (table \(\pageindex{1}\)), and the ability of chlorine to form more than one bond, means that higher fluorides of chlorine are also known, i.e., clf 3 and clf 5. fluorine, though higher than chlorine in the periodic table, has a. Chlorine Electronegative Than Fluorine.
From www.vedantu.com
Going from fluorine, chlorine, bromine to iodine the electronegativity Chlorine Electronegative Than Fluorine and yes, chlorine has a higher electron affinity than fluorine. Electronegativity is used to predict whether a bond. Thus are less attracted to the nucleus, therefore. 119 rows values for electronegativity run from 0 to 4. chlorines electrons are better shielded and so feel less nuclear charge. That is because although fluorine wants to attract. the. Chlorine Electronegative Than Fluorine.
From www.slideserve.com
PPT Q2. Explain why alkyl halides,though polar,are immiscible with Chlorine Electronegative Than Fluorine the higher electronegativity of fluorine as compared to chlorine (table \(\pageindex{1}\)), and the ability of chlorine to form more than one bond, means that higher fluorides of chlorine are also known, i.e., clf 3 and clf 5. chlorines electrons are better shielded and so feel less nuclear charge. 119 rows values for electronegativity run from 0 to. Chlorine Electronegative Than Fluorine.
From periodictable.me
How To Find Electron Configuration For Fluorine Dynamic Periodic Chlorine Electronegative Than Fluorine a) the fluorine atom is smaller than the chlorine atom, and there is less shielding from other shells of electrons. Chlorine is a bigger atom than fluorine. fluorine, though higher than chlorine in the periodic table, has a very small atomic size. why isn't chlorine as electronegative as fluorine? chlorines electrons are better shielded and so. Chlorine Electronegative Than Fluorine.
From www.youtube.com
Assertion Although fluorine is more electronegative than chlorine, p Chlorine Electronegative Than Fluorine fluorine, though higher than chlorine in the periodic table, has a very small atomic size. 1s 2 2s 2 2p x 2 2p y 2. the higher electronegativity of fluorine as compared to chlorine (table \(\pageindex{1}\)), and the ability of chlorine to form more than one bond, means that higher fluorides of chlorine are also known, i.e., clf. Chlorine Electronegative Than Fluorine.
From www.youtube.com
Fluorine is more electronegative than chlorine, yet \( \mathrm{BF Chlorine Electronegative Than Fluorine and yes, chlorine has a higher electron affinity than fluorine. That is because although fluorine wants to attract. chlorines electrons are better shielded and so feel less nuclear charge. 1s 2 2s 2 2p x 2 2p y 2. Chlorine is a bigger atom than fluorine. why isn't chlorine as electronegative as fluorine? a) the fluorine. Chlorine Electronegative Than Fluorine.
From www.ck12.org
Periodic Trends in Electronegativity CK12 Foundation Chlorine Electronegative Than Fluorine and yes, chlorine has a higher electron affinity than fluorine. the higher electronegativity of fluorine as compared to chlorine (table \(\pageindex{1}\)), and the ability of chlorine to form more than one bond, means that higher fluorides of chlorine are also known, i.e., clf 3 and clf 5. Thus are less attracted to the nucleus, therefore. 119 rows. Chlorine Electronegative Than Fluorine.
From byjus.com
why flourine is most electronegative?why not chlorine Chlorine Electronegative Than Fluorine chlorines electrons are better shielded and so feel less nuclear charge. Electronegativity is used to predict whether a bond. That is because although fluorine wants to attract. 1s 2 2s 2 2p x 2 2p y 2. and yes, chlorine has a higher electron affinity than fluorine. why isn't chlorine as electronegative as fluorine? Chlorine is a. Chlorine Electronegative Than Fluorine.
From www.toppr.com
The electron affinity (1) Of carbon is greater than oxygen (2) Of Chlorine Electronegative Than Fluorine from what i remember, the trend is that the more right and up you go on the periodic table, the more. chlorines electrons are better shielded and so feel less nuclear charge. why isn't chlorine as electronegative as fluorine? That is because although fluorine wants to attract. the higher electronegativity of fluorine as compared to chlorine. Chlorine Electronegative Than Fluorine.
From www.slideserve.com
PPT Why is Fluorine more electronegative than Carbon? PowerPoint Chlorine Electronegative Than Fluorine That is because although fluorine wants to attract. chlorines electrons are better shielded and so feel less nuclear charge. and yes, chlorine has a higher electron affinity than fluorine. 119 rows values for electronegativity run from 0 to 4. from what i remember, the trend is that the more right and up you go on the. Chlorine Electronegative Than Fluorine.
From www.slideserve.com
PPT Why is Fluorine more electronegative than Carbon? PowerPoint Chlorine Electronegative Than Fluorine 1s 2 2s 2 2p x 2 2p y 2. the higher electronegativity of fluorine as compared to chlorine (table \(\pageindex{1}\)), and the ability of chlorine to form more than one bond, means that higher fluorides of chlorine are also known, i.e., clf 3 and clf 5. That is because although fluorine wants to attract. a) the fluorine. Chlorine Electronegative Than Fluorine.
From www.alamy.com
Molecular Model of Fluorine (F2) Molecule. Vector Illustration Stock Chlorine Electronegative Than Fluorine Chlorine is a bigger atom than fluorine. Thus are less attracted to the nucleus, therefore. and yes, chlorine has a higher electron affinity than fluorine. the higher electronegativity of fluorine as compared to chlorine (table \(\pageindex{1}\)), and the ability of chlorine to form more than one bond, means that higher fluorides of chlorine are also known, i.e., clf. Chlorine Electronegative Than Fluorine.
From socratic.org
Which Chloride should have the greatest covalent character? Socratic Chlorine Electronegative Than Fluorine chlorines electrons are better shielded and so feel less nuclear charge. Thus are less attracted to the nucleus, therefore. from what i remember, the trend is that the more right and up you go on the periodic table, the more. why isn't chlorine as electronegative as fluorine? Electronegativity is used to predict whether a bond. Chlorine is. Chlorine Electronegative Than Fluorine.
From www.doubtnut.com
Although fluorine is more electronegative than chlorine but chlor Chlorine Electronegative Than Fluorine fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Thus are less attracted to the nucleus, therefore. why isn't chlorine as electronegative as fluorine? and yes, chlorine has a higher electron affinity than fluorine. a) the fluorine atom is smaller than the chlorine atom, and there is less shielding from. Chlorine Electronegative Than Fluorine.
From www.alamy.com
Molecular Model of Fluorine (F2) Molecule. Vector Illustration Stock Chlorine Electronegative Than Fluorine from what i remember, the trend is that the more right and up you go on the periodic table, the more. why isn't chlorine as electronegative as fluorine? Thus are less attracted to the nucleus, therefore. 119 rows values for electronegativity run from 0 to 4. the higher electronegativity of fluorine as compared to chlorine (table. Chlorine Electronegative Than Fluorine.
From www.youtube.com
Fluorine Has Less Electron Gain Enthalpy Than Chlorine Periodic table Chlorine Electronegative Than Fluorine fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Thus are less attracted to the nucleus, therefore. 119 rows values for electronegativity run from 0 to 4. the higher electronegativity of fluorine as compared to chlorine (table \(\pageindex{1}\)), and the ability of chlorine to form more than one bond, means that. Chlorine Electronegative Than Fluorine.
From se.dreamstime.com
Periodisk Tabell För Electronegativity Stock Illustrationer Chlorine Electronegative Than Fluorine why isn't chlorine as electronegative as fluorine? a) the fluorine atom is smaller than the chlorine atom, and there is less shielding from other shells of electrons. fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Electronegativity is used to predict whether a bond. 1s 2 2s 2 2p x 2. Chlorine Electronegative Than Fluorine.
From dxorxejky.blob.core.windows.net
Does Chlorine Have A Larger Atomic Radius Than Fluorine at Leon Cain blog Chlorine Electronegative Than Fluorine a) the fluorine atom is smaller than the chlorine atom, and there is less shielding from other shells of electrons. Chlorine is a bigger atom than fluorine. fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Electronegativity is used to predict whether a bond. why isn't chlorine as electronegative as fluorine?. Chlorine Electronegative Than Fluorine.
From maxim-has-huang.blogspot.com
Why Is Fluorine More Electronegative Than Chlorine MaximhasHuang Chlorine Electronegative Than Fluorine Electronegativity is used to predict whether a bond. the higher electronegativity of fluorine as compared to chlorine (table \(\pageindex{1}\)), and the ability of chlorine to form more than one bond, means that higher fluorides of chlorine are also known, i.e., clf 3 and clf 5. chlorines electrons are better shielded and so feel less nuclear charge. Thus are. Chlorine Electronegative Than Fluorine.