Office Of Manufacturing Quality . The office of manufacturing quality (omq) evaluates compliance with manufacturing requirements for drugs based on inspection. Learn more about the offices and divisions in fda's center for drug evaluation and research (cder) The office of manufacturing quality for drugs (omqd), pmda summarizes the business accomplishment related to gmp inspection,. Friedman is deputy director, office of manufacturing quality, which is part of the office of compliance in fda's center for drug. To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. An effective qms (“quality system”) establishes and maintains a state of control throughout the product lifecycle via systems that vigilantly. Significant findings pertinent to the quality information provided in the site dossier, the inspection team should notify the office of.
from www.collidu.com
To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. An effective qms (“quality system”) establishes and maintains a state of control throughout the product lifecycle via systems that vigilantly. Learn more about the offices and divisions in fda's center for drug evaluation and research (cder) Significant findings pertinent to the quality information provided in the site dossier, the inspection team should notify the office of. The office of manufacturing quality for drugs (omqd), pmda summarizes the business accomplishment related to gmp inspection,. The office of manufacturing quality (omq) evaluates compliance with manufacturing requirements for drugs based on inspection. Friedman is deputy director, office of manufacturing quality, which is part of the office of compliance in fda's center for drug.
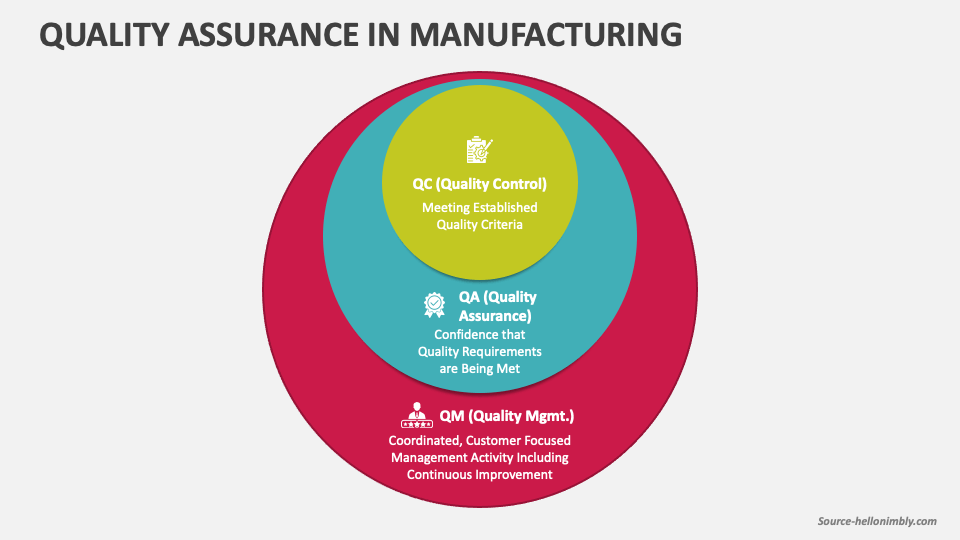
Quality Assurance In Manufacturing PowerPoint and Google Slides
Office Of Manufacturing Quality Learn more about the offices and divisions in fda's center for drug evaluation and research (cder) Learn more about the offices and divisions in fda's center for drug evaluation and research (cder) Significant findings pertinent to the quality information provided in the site dossier, the inspection team should notify the office of. The office of manufacturing quality for drugs (omqd), pmda summarizes the business accomplishment related to gmp inspection,. To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. Friedman is deputy director, office of manufacturing quality, which is part of the office of compliance in fda's center for drug. An effective qms (“quality system”) establishes and maintains a state of control throughout the product lifecycle via systems that vigilantly. The office of manufacturing quality (omq) evaluates compliance with manufacturing requirements for drugs based on inspection.
From www.hwmfg.com
Our Quality Assurance Process at H&W Manufacturing H&W Manufacturing Office Of Manufacturing Quality An effective qms (“quality system”) establishes and maintains a state of control throughout the product lifecycle via systems that vigilantly. Friedman is deputy director, office of manufacturing quality, which is part of the office of compliance in fda's center for drug. The office of manufacturing quality (omq) evaluates compliance with manufacturing requirements for drugs based on inspection. Significant findings pertinent. Office Of Manufacturing Quality.
From www.slideteam.net
Quality Plan Of Manufacturing Company Presentation PowerPoint Office Of Manufacturing Quality Significant findings pertinent to the quality information provided in the site dossier, the inspection team should notify the office of. The office of manufacturing quality for drugs (omqd), pmda summarizes the business accomplishment related to gmp inspection,. Friedman is deputy director, office of manufacturing quality, which is part of the office of compliance in fda's center for drug. Learn more. Office Of Manufacturing Quality.
From lasopaminnesota292.weebly.com
Quality objectives examples manufacturing lasopaminnesota Office Of Manufacturing Quality The office of manufacturing quality for drugs (omqd), pmda summarizes the business accomplishment related to gmp inspection,. An effective qms (“quality system”) establishes and maintains a state of control throughout the product lifecycle via systems that vigilantly. Learn more about the offices and divisions in fda's center for drug evaluation and research (cder) The office of manufacturing quality (omq) evaluates. Office Of Manufacturing Quality.
From garment.dony.vn
19 Departments And Its Functions At A Garment Factory 2023 Office Of Manufacturing Quality To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. An effective qms (“quality system”) establishes and maintains a state of control throughout the product lifecycle via systems that vigilantly. The office of manufacturing quality for drugs (omqd), pmda summarizes the business accomplishment related to gmp inspection,. Significant findings. Office Of Manufacturing Quality.
From praxie.com
Empower Your Plant Unveiling the Potential of Manufacturing Quality Office Of Manufacturing Quality An effective qms (“quality system”) establishes and maintains a state of control throughout the product lifecycle via systems that vigilantly. Significant findings pertinent to the quality information provided in the site dossier, the inspection team should notify the office of. Friedman is deputy director, office of manufacturing quality, which is part of the office of compliance in fda's center for. Office Of Manufacturing Quality.
From www.youtube.com
Quality Management Software System for Manufacturing Companies Office Of Manufacturing Quality Learn more about the offices and divisions in fda's center for drug evaluation and research (cder) Significant findings pertinent to the quality information provided in the site dossier, the inspection team should notify the office of. To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. An effective qms. Office Of Manufacturing Quality.
From www.sourceofasia.com
Source of Asia Source of Asia Source of Asia Office Of Manufacturing Quality Learn more about the offices and divisions in fda's center for drug evaluation and research (cder) The office of manufacturing quality (omq) evaluates compliance with manufacturing requirements for drugs based on inspection. To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. Friedman is deputy director, office of manufacturing. Office Of Manufacturing Quality.
From www.collidu.com
Quality Assurance In Manufacturing PowerPoint and Google Slides Office Of Manufacturing Quality Significant findings pertinent to the quality information provided in the site dossier, the inspection team should notify the office of. The office of manufacturing quality for drugs (omqd), pmda summarizes the business accomplishment related to gmp inspection,. An effective qms (“quality system”) establishes and maintains a state of control throughout the product lifecycle via systems that vigilantly. Friedman is deputy. Office Of Manufacturing Quality.
From www.plm.automation.siemens.com
Manufacturing Quality Siemens Software Office Of Manufacturing Quality Learn more about the offices and divisions in fda's center for drug evaluation and research (cder) To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. The office of manufacturing quality for drugs (omqd), pmda summarizes the business accomplishment related to gmp inspection,. Friedman is deputy director, office of. Office Of Manufacturing Quality.
From www.machinemetrics.com
Improving Quality in Automotive Manufacturing Office Of Manufacturing Quality The office of manufacturing quality (omq) evaluates compliance with manufacturing requirements for drugs based on inspection. To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. The office of manufacturing quality for drugs (omqd), pmda summarizes the business accomplishment related to gmp inspection,. Friedman is deputy director, office of. Office Of Manufacturing Quality.
From tagteammfg.com
Quality Control in a CNC Machine Shop Denver Manufacturing Office Of Manufacturing Quality The office of manufacturing quality for drugs (omqd), pmda summarizes the business accomplishment related to gmp inspection,. To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. The office of manufacturing quality (omq) evaluates compliance with manufacturing requirements for drugs based on inspection. An effective qms (“quality system”) establishes. Office Of Manufacturing Quality.
From qualityinspection.org
Factory Management the 4 Main Roles in the Quality Department Office Of Manufacturing Quality An effective qms (“quality system”) establishes and maintains a state of control throughout the product lifecycle via systems that vigilantly. The office of manufacturing quality (omq) evaluates compliance with manufacturing requirements for drugs based on inspection. Significant findings pertinent to the quality information provided in the site dossier, the inspection team should notify the office of. Learn more about the. Office Of Manufacturing Quality.
From www.europeanpharmaceuticalreview.com
Quality Assurance / Pharmaceutical Quality Systems in making medicines Office Of Manufacturing Quality Significant findings pertinent to the quality information provided in the site dossier, the inspection team should notify the office of. An effective qms (“quality system”) establishes and maintains a state of control throughout the product lifecycle via systems that vigilantly. Friedman is deputy director, office of manufacturing quality, which is part of the office of compliance in fda's center for. Office Of Manufacturing Quality.
From anvl.com
Ensuring High Quality Work in Manufacturing Anvl Office Of Manufacturing Quality An effective qms (“quality system”) establishes and maintains a state of control throughout the product lifecycle via systems that vigilantly. Significant findings pertinent to the quality information provided in the site dossier, the inspection team should notify the office of. The office of manufacturing quality for drugs (omqd), pmda summarizes the business accomplishment related to gmp inspection,. To prioritize manufacturing. Office Of Manufacturing Quality.
From edrawmind.wondershare.com
Manufacturing Company Organizational Chart EdrawMind Office Of Manufacturing Quality Learn more about the offices and divisions in fda's center for drug evaluation and research (cder) The office of manufacturing quality (omq) evaluates compliance with manufacturing requirements for drugs based on inspection. Significant findings pertinent to the quality information provided in the site dossier, the inspection team should notify the office of. To prioritize manufacturing sites (aka facilities) for routine. Office Of Manufacturing Quality.
From www.collidu.com
Quality Assurance In Manufacturing PowerPoint and Google Slides Office Of Manufacturing Quality Significant findings pertinent to the quality information provided in the site dossier, the inspection team should notify the office of. The office of manufacturing quality (omq) evaluates compliance with manufacturing requirements for drugs based on inspection. To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. Learn more about. Office Of Manufacturing Quality.
From www.augmentir.com
How to Improve Production Quality in Manufacturing Augmentir Office Of Manufacturing Quality Learn more about the offices and divisions in fda's center for drug evaluation and research (cder) To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. An effective qms (“quality system”) establishes and maintains a state of control throughout the product lifecycle via systems that vigilantly. The office of. Office Of Manufacturing Quality.
From www.dozuki.com
5 Ways To Improve Quality Control in Manufacturing Office Of Manufacturing Quality Friedman is deputy director, office of manufacturing quality, which is part of the office of compliance in fda's center for drug. The office of manufacturing quality (omq) evaluates compliance with manufacturing requirements for drugs based on inspection. An effective qms (“quality system”) establishes and maintains a state of control throughout the product lifecycle via systems that vigilantly. Learn more about. Office Of Manufacturing Quality.
From loerpsizl.blob.core.windows.net
What Is Quality Control Mechanism at Alica Williams blog Office Of Manufacturing Quality Learn more about the offices and divisions in fda's center for drug evaluation and research (cder) Significant findings pertinent to the quality information provided in the site dossier, the inspection team should notify the office of. Friedman is deputy director, office of manufacturing quality, which is part of the office of compliance in fda's center for drug. The office of. Office Of Manufacturing Quality.
From www.slideteam.net
Quality Management Systems In Manufacturing Industry PPT PowerPoint Office Of Manufacturing Quality To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. Significant findings pertinent to the quality information provided in the site dossier, the inspection team should notify the office of. The office of manufacturing quality (omq) evaluates compliance with manufacturing requirements for drugs based on inspection. An effective qms. Office Of Manufacturing Quality.
From intermetal.com
Quality Policy Intermetal Furniture Manufacturing & Designing Office Of Manufacturing Quality Learn more about the offices and divisions in fda's center for drug evaluation and research (cder) Friedman is deputy director, office of manufacturing quality, which is part of the office of compliance in fda's center for drug. The office of manufacturing quality for drugs (omqd), pmda summarizes the business accomplishment related to gmp inspection,. Significant findings pertinent to the quality. Office Of Manufacturing Quality.
From www.slidegeeks.com
Quality Assurance Templates Set 3 Quality Control Plan For Office Of Manufacturing Quality To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. The office of manufacturing quality for drugs (omqd), pmda summarizes the business accomplishment related to gmp inspection,. Learn more about the offices and divisions in fda's center for drug evaluation and research (cder) Friedman is deputy director, office of. Office Of Manufacturing Quality.
From www.slideteam.net
Pharmaceutical Quality Control And Assurance Process PPT Presentation Office Of Manufacturing Quality Significant findings pertinent to the quality information provided in the site dossier, the inspection team should notify the office of. Friedman is deputy director, office of manufacturing quality, which is part of the office of compliance in fda's center for drug. The office of manufacturing quality for drugs (omqd), pmda summarizes the business accomplishment related to gmp inspection,. The office. Office Of Manufacturing Quality.
From katanamrp.com
6 Tips for Improving Quality Control in Manufacturing — Katana Office Of Manufacturing Quality The office of manufacturing quality for drugs (omqd), pmda summarizes the business accomplishment related to gmp inspection,. Learn more about the offices and divisions in fda's center for drug evaluation and research (cder) To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. An effective qms (“quality system”) establishes. Office Of Manufacturing Quality.
From www.slideteam.net
Quality Management System In Manufacturing Company PowerPoint Office Of Manufacturing Quality To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. Significant findings pertinent to the quality information provided in the site dossier, the inspection team should notify the office of. Friedman is deputy director, office of manufacturing quality, which is part of the office of compliance in fda's center. Office Of Manufacturing Quality.
From small-bizsense.com
How to Improve Manufacturing Quality Control Small Business Sense Office Of Manufacturing Quality To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. The office of manufacturing quality for drugs (omqd), pmda summarizes the business accomplishment related to gmp inspection,. Significant findings pertinent to the quality information provided in the site dossier, the inspection team should notify the office of. Learn more. Office Of Manufacturing Quality.
From info.docxellent.com
The Four Main Components of Manufacturing Quality Management Office Of Manufacturing Quality The office of manufacturing quality for drugs (omqd), pmda summarizes the business accomplishment related to gmp inspection,. Significant findings pertinent to the quality information provided in the site dossier, the inspection team should notify the office of. To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. Friedman is. Office Of Manufacturing Quality.
From www.pinterest.com
Good Manufacturing practices yield good Quality Good manufacturing Office Of Manufacturing Quality To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. Learn more about the offices and divisions in fda's center for drug evaluation and research (cder) Significant findings pertinent to the quality information provided in the site dossier, the inspection team should notify the office of. The office of. Office Of Manufacturing Quality.
From compliancearchitects.com
Assessing Quality Culture in Manufacturing The TwoDataPoint Method Office Of Manufacturing Quality To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. Significant findings pertinent to the quality information provided in the site dossier, the inspection team should notify the office of. The office of manufacturing quality for drugs (omqd), pmda summarizes the business accomplishment related to gmp inspection,. An effective. Office Of Manufacturing Quality.
From www.qualitygurus.com
Quality Control Importance, Benefits, Approaches and Strategies Office Of Manufacturing Quality The office of manufacturing quality (omq) evaluates compliance with manufacturing requirements for drugs based on inspection. Learn more about the offices and divisions in fda's center for drug evaluation and research (cder) To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. An effective qms (“quality system”) establishes and. Office Of Manufacturing Quality.
From qmsgurus.com
Quality Assurance Reporting Structure Office Of Manufacturing Quality The office of manufacturing quality (omq) evaluates compliance with manufacturing requirements for drugs based on inspection. To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. Learn more about the offices and divisions in fda's center for drug evaluation and research (cder) The office of manufacturing quality for drugs. Office Of Manufacturing Quality.
From www.adinternationalbv.com
Quality principles AD International Office Of Manufacturing Quality The office of manufacturing quality for drugs (omqd), pmda summarizes the business accomplishment related to gmp inspection,. Friedman is deputy director, office of manufacturing quality, which is part of the office of compliance in fda's center for drug. To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. Learn. Office Of Manufacturing Quality.
From moellerpunch.com
Quality Control for Precision Tooling Explained Office Of Manufacturing Quality To prioritize manufacturing sites (aka facilities) for routine cgmp surveillance inspections.* these inspections ensure that sites consistently manufacture drug products of. Significant findings pertinent to the quality information provided in the site dossier, the inspection team should notify the office of. The office of manufacturing quality (omq) evaluates compliance with manufacturing requirements for drugs based on inspection. Friedman is deputy. Office Of Manufacturing Quality.
From www.getreskilled.com
Departments in a Pharmaceutical Manufacturing Company Office Of Manufacturing Quality An effective qms (“quality system”) establishes and maintains a state of control throughout the product lifecycle via systems that vigilantly. The office of manufacturing quality for drugs (omqd), pmda summarizes the business accomplishment related to gmp inspection,. Significant findings pertinent to the quality information provided in the site dossier, the inspection team should notify the office of. Learn more about. Office Of Manufacturing Quality.
From www.slideteam.net
Quality Control Process Flowchart For Manufacturing Company PPT Example Office Of Manufacturing Quality An effective qms (“quality system”) establishes and maintains a state of control throughout the product lifecycle via systems that vigilantly. Learn more about the offices and divisions in fda's center for drug evaluation and research (cder) The office of manufacturing quality for drugs (omqd), pmda summarizes the business accomplishment related to gmp inspection,. Friedman is deputy director, office of manufacturing. Office Of Manufacturing Quality.