Buffers Lab Report . Define a buffer and explain how a buffer works. The professor used naoh (a base) to raise the ph to the desired. Calculation of buffer components the amount of acid and conjugate base components of the buffer is determined using the calculation below. 1) prepare a buffer solution at a given ph and concentration. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. Which reagent was used to adjust the ph of the buffer solution? There is not a formal lab report for this lab. Briefly describe what a buffer is. The prepared buffer solution ph was lower than the desired ph. Upon completion of this lab, the student will be able to: Complete the below pages and submit them to your ta before leaving lab. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. Designing and preparing a buffer. A buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph unit of pka.
from www.studocu.com
Designing and preparing a buffer. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. The professor used naoh (a base) to raise the ph to the desired. Which reagent was used to adjust the ph of the buffer solution? Calculation of buffer components the amount of acid and conjugate base components of the buffer is determined using the calculation below. Briefly describe what a buffer is. Upon completion of this lab, the student will be able to: Complete the below pages and submit them to your ta before leaving lab. The prepared buffer solution ph was lower than the desired ph. There is not a formal lab report for this lab.
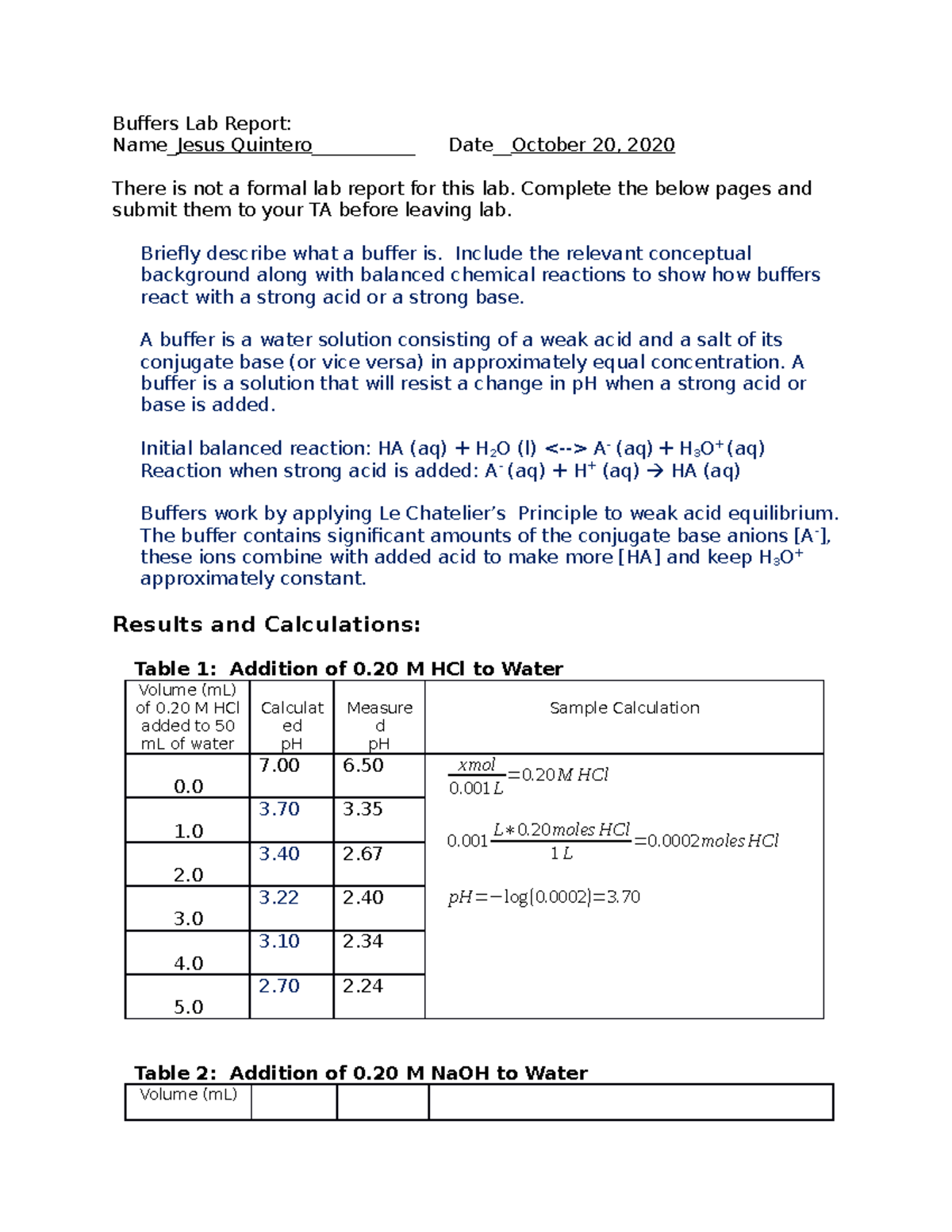
Buffers Lab 4 Lab 4 Buffers Lab Report Name_Jesus Quintero
Buffers Lab Report Upon completion of this lab, the student will be able to: Upon completion of this lab, the student will be able to: Briefly describe what a buffer is. Designing and preparing a buffer. The professor used naoh (a base) to raise the ph to the desired. The prepared buffer solution ph was lower than the desired ph. Calculation of buffer components the amount of acid and conjugate base components of the buffer is determined using the calculation below. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. Define a buffer and explain how a buffer works. Which reagent was used to adjust the ph of the buffer solution? There is not a formal lab report for this lab. Complete the below pages and submit them to your ta before leaving lab. A buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph unit of pka. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. 1) prepare a buffer solution at a given ph and concentration.
From www.studocu.com
Experiment 4 Buffer Lab Report Chemistry 241L (Sections Studocu Buffers Lab Report The professor used naoh (a base) to raise the ph to the desired. Designing and preparing a buffer. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. Calculation of buffer components the amount of acid and conjugate base components of the buffer is determined using. Buffers Lab Report.
From www.studocu.com
Lab 2 Chem lab Lab 2 Chem lab CHEMISTRY LABORATORY REPORT Buffers Lab Report 1) prepare a buffer solution at a given ph and concentration. The prepared buffer solution ph was lower than the desired ph. Designing and preparing a buffer. A buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph unit of pka. Briefly describe what a buffer is. There is. Buffers Lab Report.
From www.studocu.com
Lab 2 The scientific Method Buffer Lab 2 Buffers. Worth = (points Buffers Lab Report Briefly describe what a buffer is. Calculation of buffer components the amount of acid and conjugate base components of the buffer is determined using the calculation below. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. Which reagent was used to adjust the ph of. Buffers Lab Report.
From www.chegg.com
Solved Unit IX Buffer Solutions LAB REPORT Name Date Buffers Lab Report Designing and preparing a buffer. Calculation of buffer components the amount of acid and conjugate base components of the buffer is determined using the calculation below. Which reagent was used to adjust the ph of the buffer solution? Briefly describe what a buffer is. The prepared buffer solution ph was lower than the desired ph. Define a buffer and explain. Buffers Lab Report.
From www.transtutors.com
(Solved) REPORT SHEET LAB Acids, Bases, pH, and Buffers 19 A Buffers Lab Report Upon completion of this lab, the student will be able to: The prepared buffer solution ph was lower than the desired ph. 1) prepare a buffer solution at a given ph and concentration. Designing and preparing a buffer. Which reagent was used to adjust the ph of the buffer solution? • investigate the nature of buffer solutions with respect to. Buffers Lab Report.
From www.studypool.com
SOLUTION Biochemistry laboratory final report buffer system acid base Buffers Lab Report Define a buffer and explain how a buffer works. A buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph unit of pka. Upon completion of this lab, the student will be able to: Complete the below pages and submit them to your ta before leaving lab. • investigate. Buffers Lab Report.
From www.scribd.com
Lab Report 1 Buffer Solution Ph Buffers Lab Report The prepared buffer solution ph was lower than the desired ph. Complete the below pages and submit them to your ta before leaving lab. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. The dissociation of a weak acid in water yields h+ ion and. Buffers Lab Report.
From www.studocu.com
Buffer Lab report summary Section______________ CHM115L Buffers Lab Buffers Lab Report The professor used naoh (a base) to raise the ph to the desired. Which reagent was used to adjust the ph of the buffer solution? The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. A buffer solution has the greatest capacity when the concentration ratio of the two components. Buffers Lab Report.
From www.studypool.com
SOLUTION Ph and buffer system Lab Report Studypool Buffers Lab Report Define a buffer and explain how a buffer works. The prepared buffer solution ph was lower than the desired ph. The professor used naoh (a base) to raise the ph to the desired. There is not a formal lab report for this lab. Upon completion of this lab, the student will be able to: A buffer solution has the greatest. Buffers Lab Report.
From www.chegg.com
Solved BUFFERS, TITRATION CURVES, AND INDICATORS LAB REPORT Buffers Lab Report Define a buffer and explain how a buffer works. 1) prepare a buffer solution at a given ph and concentration. Upon completion of this lab, the student will be able to: Briefly describe what a buffer is. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. Complete the below. Buffers Lab Report.
From www.studypool.com
SOLUTION Lab report pH and Buffers Studypool Buffers Lab Report Designing and preparing a buffer. The professor used naoh (a base) to raise the ph to the desired. Briefly describe what a buffer is. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. Define a buffer and explain how a buffer works. The prepared buffer solution ph was lower. Buffers Lab Report.
From www.studocu.com
Report Expt GENERAL CHEMISTRY LABORATORY REPORT EXPERIMENT 2 pH AND Buffers Lab Report The professor used naoh (a base) to raise the ph to the desired. Which reagent was used to adjust the ph of the buffer solution? • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. A buffer solution has the greatest capacity when the concentration ratio. Buffers Lab Report.
From www.studocu.com
Buffers PreLab Chemistry preLab report Buffers Purpose To Buffers Lab Report Complete the below pages and submit them to your ta before leaving lab. 1) prepare a buffer solution at a given ph and concentration. Upon completion of this lab, the student will be able to: Define a buffer and explain how a buffer works. The dissociation of a weak acid in water yields h+ ion and the conjugate base of. Buffers Lab Report.
From www.studocu.com
Report 2 p H AND Buffers lab 2 CHEMISTRY LABORATORY REPORT Buffers Lab Report Upon completion of this lab, the student will be able to: Briefly describe what a buffer is. Calculation of buffer components the amount of acid and conjugate base components of the buffer is determined using the calculation below. The professor used naoh (a base) to raise the ph to the desired. A buffer solution has the greatest capacity when the. Buffers Lab Report.
From www.studypool.com
SOLUTION Lab report ph and buffers Studypool Buffers Lab Report Complete the below pages and submit them to your ta before leaving lab. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. There is not a formal lab report for this lab. The professor used naoh (a base) to raise the ph to the desired. Define a buffer and. Buffers Lab Report.
From www.studocu.com
Lab report 2 CHEM271 EXPERIMENT 2 BUFFERS AND BIOCHEMICAL ANALYSIS Buffers Lab Report • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. The prepared buffer solution ph was lower than the desired ph. Define a buffer and explain. Buffers Lab Report.
From www.studypool.com
SOLUTION Ph and buffer system Lab Report Studypool Buffers Lab Report The professor used naoh (a base) to raise the ph to the desired. Designing and preparing a buffer. The prepared buffer solution ph was lower than the desired ph. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. Calculation of buffer components the amount of acid and conjugate base. Buffers Lab Report.
From www.studocu.com
Buffers Lab 4 Lab 4 Buffers Lab Report Name_Jesus Quintero Buffers Lab Report 1) prepare a buffer solution at a given ph and concentration. Upon completion of this lab, the student will be able to: Complete the below pages and submit them to your ta before leaving lab. The professor used naoh (a base) to raise the ph to the desired. The dissociation of a weak acid in water yields h+ ion and. Buffers Lab Report.
From www.studypool.com
SOLUTION Biochem ph and buffers laboratory report Studypool Buffers Lab Report • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. Complete the below pages and submit them to your ta before leaving lab. 1) prepare a buffer solution at a given ph and concentration. Which reagent was used to adjust the ph of the buffer solution?. Buffers Lab Report.
From www.chegg.com
Solved REPORT SHEET LAB Acids, Bases, pH, and Buffers 19 PH Buffers Lab Report A buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph unit of pka. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. Designing and preparing a buffer. Which reagent was used to adjust. Buffers Lab Report.
From www.chegg.com
Solved REPORT SHEET LAB Acids, Bases, pH, and Buffers 19 PH Buffers Lab Report Calculation of buffer components the amount of acid and conjugate base components of the buffer is determined using the calculation below. Briefly describe what a buffer is. Define a buffer and explain how a buffer works. Designing and preparing a buffer. 1) prepare a buffer solution at a given ph and concentration. Which reagent was used to adjust the ph. Buffers Lab Report.
From www.chegg.com
Solved Unit IX Buffer Solutions LAB REPORT Name Date Buffers Lab Report There is not a formal lab report for this lab. The prepared buffer solution ph was lower than the desired ph. Which reagent was used to adjust the ph of the buffer solution? Briefly describe what a buffer is. 1) prepare a buffer solution at a given ph and concentration. Upon completion of this lab, the student will be able. Buffers Lab Report.
From www.studocu.com
Working with buffers Lab Report BRAYAM CARDONA TA MARIO VENDRELL Buffers Lab Report Upon completion of this lab, the student will be able to: Calculation of buffer components the amount of acid and conjugate base components of the buffer is determined using the calculation below. Which reagent was used to adjust the ph of the buffer solution? Briefly describe what a buffer is. There is not a formal lab report for this lab.. Buffers Lab Report.
From www.studypool.com
SOLUTION Lab report ph and buffers Studypool Buffers Lab Report • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. Briefly describe what a buffer is. Which reagent was used to adjust the ph of the buffer solution? Calculation of buffer components the amount of acid and conjugate base components of the buffer is determined using. Buffers Lab Report.
From www.studocu.com
Lab 7 Buffers lab report Gianna Puopolo Lab Partner Nila Murugan Buffers Lab Report Upon completion of this lab, the student will be able to: Briefly describe what a buffer is. Complete the below pages and submit them to your ta before leaving lab. Calculation of buffer components the amount of acid and conjugate base components of the buffer is determined using the calculation below. • investigate the nature of buffer solutions with respect. Buffers Lab Report.
From www.studypool.com
SOLUTION Lab report ph and buffers Studypool Buffers Lab Report • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. A buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph unit of pka. Define a buffer and explain how a buffer works. Which reagent. Buffers Lab Report.
From www.studypool.com
SOLUTION Lab report buffers Studypool Buffers Lab Report Which reagent was used to adjust the ph of the buffer solution? Briefly describe what a buffer is. The professor used naoh (a base) to raise the ph to the desired. Designing and preparing a buffer. Complete the below pages and submit them to your ta before leaving lab. Calculation of buffer components the amount of acid and conjugate base. Buffers Lab Report.
From www.studocu.com
Buffers lab 1 \ Lab Buffers Abstract The purpose of this lab was to Buffers Lab Report There is not a formal lab report for this lab. The prepared buffer solution ph was lower than the desired ph. The professor used naoh (a base) to raise the ph to the desired. Briefly describe what a buffer is. A buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within. Buffers Lab Report.
From studylib.net
Lab 19 Buffers Buffers Lab Report Upon completion of this lab, the student will be able to: 1) prepare a buffer solution at a given ph and concentration. The prepared buffer solution ph was lower than the desired ph. There is not a formal lab report for this lab. The professor used naoh (a base) to raise the ph to the desired. Briefly describe what a. Buffers Lab Report.
From www.studocu.com
P H Measurement 313 lab report pH Measurement and its Applications Buffers Lab Report The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. Which reagent was used to adjust the ph of the buffer solution? Designing and preparing a buffer. 1) prepare a buffer solution at a given ph and concentration. A buffer solution has the greatest capacity when the concentration ratio of. Buffers Lab Report.
From www.chegg.com
Solved pH and Buffer lab reportI need help with question Buffers Lab Report Which reagent was used to adjust the ph of the buffer solution? A buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph unit of pka. There is not a formal lab report for this lab. The dissociation of a weak acid in water yields h+ ion and the. Buffers Lab Report.
From www.studocu.com
Lab 05 p H and Buffers Lab report. Lab pH and Buffers Buffers Lab Report Define a buffer and explain how a buffer works. Calculation of buffer components the amount of acid and conjugate base components of the buffer is determined using the calculation below. Designing and preparing a buffer. The prepared buffer solution ph was lower than the desired ph. A buffer solution has the greatest capacity when the concentration ratio of the two. Buffers Lab Report.
From www.chegg.com
Solved Experiment VII Buffers Lab Report ( 49 pts) I. Buffers Lab Report The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. Define a buffer and explain how a buffer works. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. A buffer solution has the greatest capacity. Buffers Lab Report.
From www.chegg.com
Solved PreLab Investigation 11 How does the buffer Buffers Lab Report Complete the below pages and submit them to your ta before leaving lab. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. The prepared buffer solution ph was lower than the desired ph. Briefly describe what a buffer is. There is not a formal lab. Buffers Lab Report.
From www.studocu.com
Lab 3 report Laboratory N° 3 Biological Buffers IIPreparation of a Buffers Lab Report The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. The prepared buffer solution ph was lower than the desired ph. Upon completion of this lab, the student will be able to: 1) prepare a buffer solution at a given ph and concentration. • investigate the nature of buffer solutions. Buffers Lab Report.