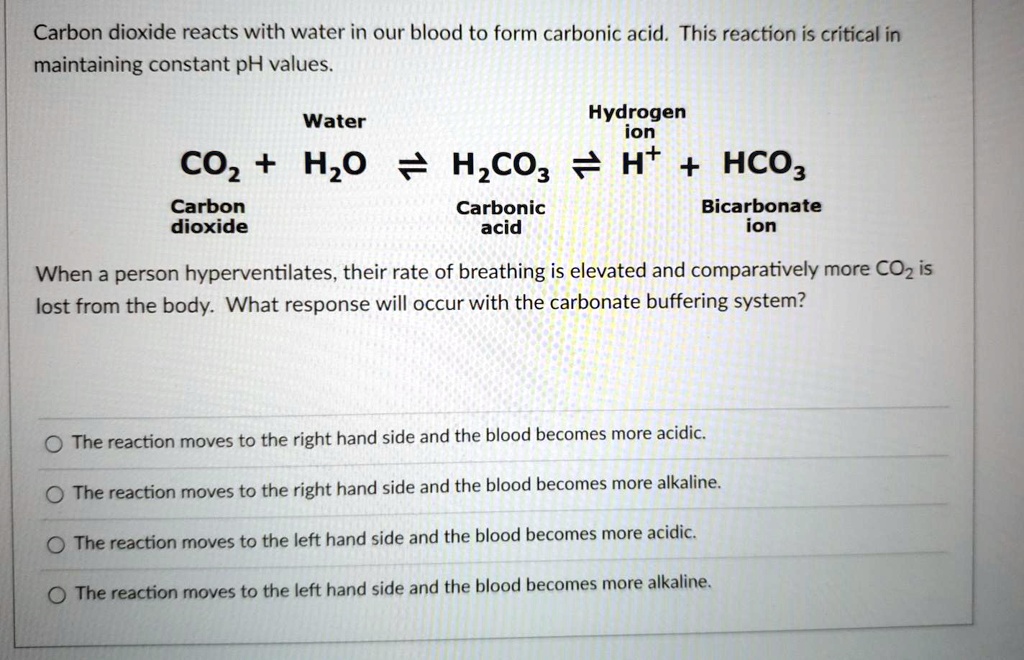
Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood . Carbonic acid then dissociates into bicarbonate and a hydrogen ion. In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The carbon dioxide formed during cellular respiration combines with water to create carbonic acid. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.
from www.numerade.com
The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. In this system, gaseous metabolic waste carbon dioxide reacts. The carbon dioxide formed during cellular respiration combines with water to create carbonic acid. Carbonic acid then dissociates into bicarbonate and a hydrogen ion.
SOLVED Carbon dioxide reacts with water in our blood to form carbonic
Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Carbonic acid then dissociates into bicarbonate and a hydrogen ion. The carbon dioxide formed during cellular respiration combines with water to create carbonic acid. In this system, gaseous metabolic waste carbon dioxide reacts. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.
From www.pinterest.ca
bicarbonate buffer system, example of multiple equilibria Teaching Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The carbon dioxide formed during cellular respiration combines. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The carbon dioxide formed during cellular respiration combines with water to create carbonic acid. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. Carbonic acid then. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.pinterest.com
Image result for bicarbonate buffer system Medical student motivation Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood Carbonic acid then dissociates into bicarbonate and a hydrogen ion. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. In this system, gaseous metabolic waste carbon dioxide reacts. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.slideshare.net
Buffer system Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this system, gaseous metabolic waste carbon dioxide reacts. Carbonic acid then dissociates into bicarbonate and a hydrogen ion. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The carbon dioxide formed during cellular respiration. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From quizlet.com
Bicarbonate Buffer system Diagram Quizlet Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood The carbon dioxide formed during cellular respiration combines with water to create carbonic acid. In this system, gaseous metabolic waste carbon dioxide reacts. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −). Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.youtube.com
Buffer action in the blood YouTube Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood In this system, gaseous metabolic waste carbon dioxide reacts. Carbonic acid then dissociates into bicarbonate and a hydrogen ion. The carbon dioxide formed during cellular respiration combines with water to create carbonic acid. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood contains a buffer of carbonic acid (h2co3 h. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From resources.wfsahq.org
Respiratory Physiology Part 2 WFSA Resources Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood The carbon dioxide formed during cellular respiration combines with water to create carbonic acid. In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate,. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From study.com
Bicarbonate Buffer System Overview, Equation & Uses Video & Lesson Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood In this system, gaseous metabolic waste carbon dioxide reacts. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Carbonic acid then dissociates into bicarbonate and a hydrogen ion. The carbon dioxide formed during cellular respiration combines with water to create carbonic acid. Human blood contains a buffer of carbonic acid (h2co3 h. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.coursehero.com
[Solved] Describe the blood hydrogen carbonate buffer system. What Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The carbon dioxide formed during cellular respiration combines with water to create carbonic acid. Carbonic acid then dissociates into bicarbonate and a hydrogen ion. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.numerade.com
SOLVED One of the buffers that contribute to pH stability in human Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood Carbonic acid then dissociates into bicarbonate and a hydrogen ion. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this system, gaseous metabolic waste carbon dioxide reacts. The carbon dioxide formed during cellular respiration. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From slideplayer.com
2 The Chemical Context of Life. ppt download Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood In this system, gaseous metabolic waste carbon dioxide reacts. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The buffer systems functioning in blood plasma include. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From ar.inspiredpencil.com
Renal Buffer System Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Carbonic acid then dissociates into bicarbonate and a hydrogen ion. The buffer systems functioning in blood plasma. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.numerade.com
SOLVED Carbonic acid has a pKa of 6.1 at physiological temperature. Is Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood Carbonic acid then dissociates into bicarbonate and a hydrogen ion. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The carbon dioxide formed during cellular respiration. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.numerade.com
SOLVED The normal pH of human blood is about 7.4. The carbonate buffer Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The carbon dioxide formed during cellular respiration combines with water to create carbonic acid. In this system,. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.slideshare.net
11 acid base regulation Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood Carbonic acid then dissociates into bicarbonate and a hydrogen ion. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The buffer systems functioning in blood plasma. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From mavink.com
Hydrogen Carbonate Lewis Structure Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood The carbon dioxide formed during cellular respiration combines with water to create carbonic acid. In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate,. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From lindsay-kbeard.blogspot.com
Describe a System Buffered With Carbonic Acid Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Carbonic acid then dissociates into bicarbonate and a hydrogen ion. The carbon dioxide formed during cellular respiration combines with water to create carbonic acid. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From books.byui.edu
Oxygen and Carbon Dioxide Transport in the Blood Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.visionlearning.com
Acids and Bases II Chemistry Visionlearning Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. Carbonic acid then dissociates into bicarbonate and a hydrogen ion. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The buffer systems functioning in blood plasma. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.slideserve.com
PPT Chapter 27, part 2 PowerPoint Presentation, free download ID Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood The carbon dioxide formed during cellular respiration combines with water to create carbonic acid. In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate,. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation, free Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood In this system, gaseous metabolic waste carbon dioxide reacts. The carbon dioxide formed during cellular respiration combines with water to create carbonic acid. Carbonic acid then dissociates into bicarbonate and a hydrogen ion. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.slideserve.com
PPT Acidbase homeostasis PowerPoint Presentation, free download ID Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this system, gaseous metabolic waste carbon dioxide reacts. Carbonic acid then dissociates into bicarbonate and a. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.slideserve.com
PPT RESPIRATORY SYSTEM 3 PowerPoint Presentation, free download Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this system, gaseous metabolic waste carbon dioxide reacts. Carbonic acid then dissociates into bicarbonate and a hydrogen ion. Human blood contains a buffer of carbonic. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.numerade.com
SOLVED Carbon dioxide reacts with water in our blood to form carbonic Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The carbon dioxide formed during cellular respiration combines with water to create carbonic acid. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Carbonic acid then dissociates into bicarbonate and a hydrogen ion. Human blood. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.slideserve.com
PPT Chapter 27, part 2 PowerPoint Presentation, free download ID Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The carbon dioxide formed during cellular respiration combines with water to create carbonic acid. The buffer systems functioning in blood plasma include plasma proteins, phosphate,. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From slideplayer.com
Buffers A buffer is a mixture of chemicals that make a solution resist Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. Carbonic acid then dissociates into bicarbonate and a hydrogen ion. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this system, gaseous metabolic waste carbon. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.slideserve.com
PPT ARTERIAL BLOOD GAS ANALYSIS PowerPoint Presentation ID5086981 Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this system, gaseous metabolic waste carbon dioxide reacts. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The carbon dioxide formed during cellular respiration combines with water to create carbonic acid. Human blood contains. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.slideserve.com
PPT Chapter 27 Fluid, Electrolyte and Acidbase Balance PowerPoint Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood The carbon dioxide formed during cellular respiration combines with water to create carbonic acid. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. Carbonic acid then dissociates into bicarbonate and a hydrogen ion. The buffer systems functioning in blood plasma include plasma proteins,. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.pinterest.com.au
Pin on Paramedici Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The carbon dioxide formed during cellular respiration combines with water to create carbonic acid. In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −). Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.reagent.co.uk
How Do Biological Buffers Work? The Science Blog Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The carbon dioxide formed during cellular respiration combines. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.pinterest.com
Bicarbonate Buffer System bodybuildingdesign Nursing school studying Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood Carbonic acid then dissociates into bicarbonate and a hydrogen ion. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The carbon dioxide formed during cellular respiration. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From labpedia.net
Acidbase Balance Part 2 Metabolic acidosis, and Metabolic Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood The carbon dioxide formed during cellular respiration combines with water to create carbonic acid. In this system, gaseous metabolic waste carbon dioxide reacts. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Carbonic acid then. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From animalia-life.club
Phosphate Buffer System Equation Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood In this system, gaseous metabolic waste carbon dioxide reacts. Carbonic acid then dissociates into bicarbonate and a hydrogen ion. The carbon dioxide formed during cellular respiration combines with water to create carbonic acid. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Human blood contains a buffer of carbonic acid (h2co3 h. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The carbon dioxide formed during cellular respiration combines with water to create carbonic acid. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. Carbonic acid then. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.
From www.linstitute.net
CIE A Level Biology复习笔记8.2.3 Plasma & Carbon Dioxide翰林国际教育 Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this system, gaseous metabolic waste carbon dioxide reacts. Carbonic acid then dissociates into bicarbonate and a. Carbonic Acid/Hydrogen Carbonate Buffer System In Human Blood.