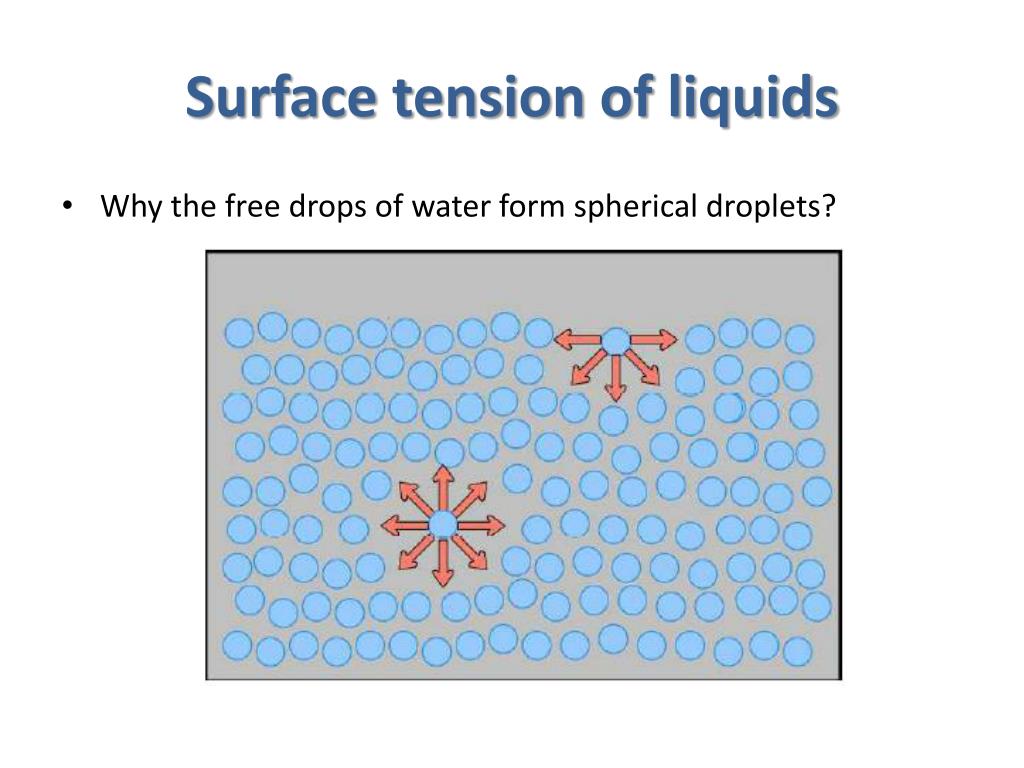
Effects Of Surface Tension . Let’s consider the effects of these four conditions on surface tension: “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Why does surface tension decrease with increasing temperature? This effect results from the difference between the potential energy. As the molecules get agitated, they. As temperature decreases, surface tension increase s. The further the temperature increases we can. Let’s consider the effects of these four conditions on surface tension: As temperature decreases, surface tension increases. The surface tension is the reversible work per unit area needed to elastically stretch/compress a preexisting surface. Increasing the temperature increases the kinetic energy of the molecules. Thus, surface tension forces play a role in its floating behaviour, and the resulting value of h changes depending on whether surface tension is considered.
from www.slideserve.com
As the molecules get agitated, they. As temperature decreases, surface tension increases. Increasing the temperature increases the kinetic energy of the molecules. The further the temperature increases we can. This effect results from the difference between the potential energy. Let’s consider the effects of these four conditions on surface tension: As temperature decreases, surface tension increase s. Why does surface tension decrease with increasing temperature? “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Thus, surface tension forces play a role in its floating behaviour, and the resulting value of h changes depending on whether surface tension is considered.
PPT Physical Pharmacy SURFACE TENSION PowerPoint Presentation, free
Effects Of Surface Tension Why does surface tension decrease with increasing temperature? This effect results from the difference between the potential energy. As temperature decreases, surface tension increases. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The further the temperature increases we can. Increasing the temperature increases the kinetic energy of the molecules. The surface tension is the reversible work per unit area needed to elastically stretch/compress a preexisting surface. Let’s consider the effects of these four conditions on surface tension: As temperature decreases, surface tension increase s. Let’s consider the effects of these four conditions on surface tension: Thus, surface tension forces play a role in its floating behaviour, and the resulting value of h changes depending on whether surface tension is considered. As the molecules get agitated, they. Why does surface tension decrease with increasing temperature?
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID9168408 Effects Of Surface Tension Let’s consider the effects of these four conditions on surface tension: As temperature decreases, surface tension increase s. This effect results from the difference between the potential energy. As the molecules get agitated, they. Let’s consider the effects of these four conditions on surface tension: Increasing the temperature increases the kinetic energy of the molecules. Why does surface tension decrease. Effects Of Surface Tension.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID3227401 Effects Of Surface Tension Thus, surface tension forces play a role in its floating behaviour, and the resulting value of h changes depending on whether surface tension is considered. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. As the molecules get. Effects Of Surface Tension.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog Effects Of Surface Tension This effect results from the difference between the potential energy. The further the temperature increases we can. Thus, surface tension forces play a role in its floating behaviour, and the resulting value of h changes depending on whether surface tension is considered. The surface tension is the reversible work per unit area needed to elastically stretch/compress a preexisting surface. Let’s. Effects Of Surface Tension.
From www.scribd.com
Explore The Concept of Surface Tension and Its Effects On Different Effects Of Surface Tension Thus, surface tension forces play a role in its floating behaviour, and the resulting value of h changes depending on whether surface tension is considered. As the molecules get agitated, they. As temperature decreases, surface tension increases. Let’s consider the effects of these four conditions on surface tension: This effect results from the difference between the potential energy. Let’s consider. Effects Of Surface Tension.
From www.biolinscientific.com
3 ways to measure surface tension Effects Of Surface Tension Let’s consider the effects of these four conditions on surface tension: The further the temperature increases we can. Thus, surface tension forces play a role in its floating behaviour, and the resulting value of h changes depending on whether surface tension is considered. As the molecules get agitated, they. Increasing the temperature increases the kinetic energy of the molecules. Let’s. Effects Of Surface Tension.
From www.researchgate.net
Influence of surface tension gradients of liquidgallium on the Effects Of Surface Tension As temperature decreases, surface tension increases. Increasing the temperature increases the kinetic energy of the molecules. Thus, surface tension forces play a role in its floating behaviour, and the resulting value of h changes depending on whether surface tension is considered. This effect results from the difference between the potential energy. “surface tension is the tension of a liquid’s surface. Effects Of Surface Tension.
From askfilo.com
\ 6 \rightarrow What is surface tension of liquids? Explain the effect o.. Effects Of Surface Tension Why does surface tension decrease with increasing temperature? As the molecules get agitated, they. The further the temperature increases we can. As temperature decreases, surface tension increase s. The surface tension is the reversible work per unit area needed to elastically stretch/compress a preexisting surface. “surface tension is the tension of a liquid’s surface film caused by the bulk of. Effects Of Surface Tension.
From www.slideserve.com
PPT ThermoFluid PowerPoint Presentation, free download ID4559046 Effects Of Surface Tension Thus, surface tension forces play a role in its floating behaviour, and the resulting value of h changes depending on whether surface tension is considered. This effect results from the difference between the potential energy. Why does surface tension decrease with increasing temperature? Increasing the temperature increases the kinetic energy of the molecules. The surface tension is the reversible work. Effects Of Surface Tension.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 Effects Of Surface Tension As temperature decreases, surface tension increase s. Thus, surface tension forces play a role in its floating behaviour, and the resulting value of h changes depending on whether surface tension is considered. Increasing the temperature increases the kinetic energy of the molecules. Why does surface tension decrease with increasing temperature? As the molecules get agitated, they. As temperature decreases, surface. Effects Of Surface Tension.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Effects Of Surface Tension As temperature decreases, surface tension increases. As temperature decreases, surface tension increase s. Increasing the temperature increases the kinetic energy of the molecules. Let’s consider the effects of these four conditions on surface tension: This effect results from the difference between the potential energy. Thus, surface tension forces play a role in its floating behaviour, and the resulting value of. Effects Of Surface Tension.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) Effects Of Surface Tension “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Increasing the temperature increases the kinetic energy of the molecules. The further the temperature increases we can. Thus, surface tension forces play a role in its floating behaviour, and. Effects Of Surface Tension.
From www.researchgate.net
Effects of surface tension on bubble diameter at constant gas mass flow Effects Of Surface Tension Let’s consider the effects of these four conditions on surface tension: The surface tension is the reversible work per unit area needed to elastically stretch/compress a preexisting surface. The further the temperature increases we can. Increasing the temperature increases the kinetic energy of the molecules. Why does surface tension decrease with increasing temperature? As temperature decreases, surface tension increases. Let’s. Effects Of Surface Tension.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and Effects Of Surface Tension As temperature decreases, surface tension increase s. Let’s consider the effects of these four conditions on surface tension: Why does surface tension decrease with increasing temperature? As the molecules get agitated, they. The surface tension is the reversible work per unit area needed to elastically stretch/compress a preexisting surface. The further the temperature increases we can. Increasing the temperature increases. Effects Of Surface Tension.
From byjus.com
Explain the surface tension phenomenon with examples. Effects Of Surface Tension As the molecules get agitated, they. As temperature decreases, surface tension increases. As temperature decreases, surface tension increase s. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. This effect results from the difference between the potential energy.. Effects Of Surface Tension.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint Effects Of Surface Tension As temperature decreases, surface tension increases. The surface tension is the reversible work per unit area needed to elastically stretch/compress a preexisting surface. Increasing the temperature increases the kinetic energy of the molecules. Thus, surface tension forces play a role in its floating behaviour, and the resulting value of h changes depending on whether surface tension is considered. Why does. Effects Of Surface Tension.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy Effects Of Surface Tension “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. This effect results from the difference between the potential energy. Increasing the temperature increases the kinetic energy of the molecules. Thus, surface tension forces play a role in its. Effects Of Surface Tension.
From funsizephysics.com
What is Surface Tension? FunsizePhysics Effects Of Surface Tension Let’s consider the effects of these four conditions on surface tension: Increasing the temperature increases the kinetic energy of the molecules. As the molecules get agitated, they. Let’s consider the effects of these four conditions on surface tension: “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in. Effects Of Surface Tension.
From www.youtube.com
What is Effect Of Temperature on surface tension Properties of Effects Of Surface Tension Let’s consider the effects of these four conditions on surface tension: As the molecules get agitated, they. The further the temperature increases we can. As temperature decreases, surface tension increase s. Thus, surface tension forces play a role in its floating behaviour, and the resulting value of h changes depending on whether surface tension is considered. Increasing the temperature increases. Effects Of Surface Tension.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids Effects Of Surface Tension Let’s consider the effects of these four conditions on surface tension: As temperature decreases, surface tension increases. The surface tension is the reversible work per unit area needed to elastically stretch/compress a preexisting surface. Thus, surface tension forces play a role in its floating behaviour, and the resulting value of h changes depending on whether surface tension is considered. As. Effects Of Surface Tension.
From www.slideserve.com
PPT Heat Transfer in a LiquidSolid Circulating Fluidized Bed Reactor Effects Of Surface Tension The surface tension is the reversible work per unit area needed to elastically stretch/compress a preexisting surface. Let’s consider the effects of these four conditions on surface tension: As temperature decreases, surface tension increases. This effect results from the difference between the potential energy. As temperature decreases, surface tension increase s. “surface tension is the tension of a liquid’s surface. Effects Of Surface Tension.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Effects Of Surface Tension Why does surface tension decrease with increasing temperature? Thus, surface tension forces play a role in its floating behaviour, and the resulting value of h changes depending on whether surface tension is considered. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends. Effects Of Surface Tension.
From www.moleaer.com
How nanobubbles improve water infiltration by reducing surface tension Effects Of Surface Tension Thus, surface tension forces play a role in its floating behaviour, and the resulting value of h changes depending on whether surface tension is considered. This effect results from the difference between the potential energy. Let’s consider the effects of these four conditions on surface tension: As temperature decreases, surface tension increase s. Increasing the temperature increases the kinetic energy. Effects Of Surface Tension.
From www.pcimag.com
Solving Film Defects with Surface Tension Modifiers 20160401 PCI Effects Of Surface Tension As the molecules get agitated, they. Increasing the temperature increases the kinetic energy of the molecules. This effect results from the difference between the potential energy. Let’s consider the effects of these four conditions on surface tension: The further the temperature increases we can. Why does surface tension decrease with increasing temperature? Thus, surface tension forces play a role in. Effects Of Surface Tension.
From www.alamy.com
Surface tension hires stock photography and images Alamy Effects Of Surface Tension Let’s consider the effects of these four conditions on surface tension: Thus, surface tension forces play a role in its floating behaviour, and the resulting value of h changes depending on whether surface tension is considered. As temperature decreases, surface tension increase s. This effect results from the difference between the potential energy. The further the temperature increases we can.. Effects Of Surface Tension.
From www.vrogue.co
Surface Tension Definition Examples And Unit vrogue.co Effects Of Surface Tension Why does surface tension decrease with increasing temperature? The surface tension is the reversible work per unit area needed to elastically stretch/compress a preexisting surface. Let’s consider the effects of these four conditions on surface tension: Increasing the temperature increases the kinetic energy of the molecules. This effect results from the difference between the potential energy. As temperature decreases, surface. Effects Of Surface Tension.
From proper-cooking.info
Surface Tension Examples In Nature Effects Of Surface Tension The further the temperature increases we can. Let’s consider the effects of these four conditions on surface tension: As temperature decreases, surface tension increases. As the molecules get agitated, they. As temperature decreases, surface tension increase s. Let’s consider the effects of these four conditions on surface tension: Increasing the temperature increases the kinetic energy of the molecules. Why does. Effects Of Surface Tension.
From enggvacancy07.blogspot.com
Surface tension capillarity Effects Effects Of Surface Tension Increasing the temperature increases the kinetic energy of the molecules. Let’s consider the effects of these four conditions on surface tension: The surface tension is the reversible work per unit area needed to elastically stretch/compress a preexisting surface. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in. Effects Of Surface Tension.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii Effects Of Surface Tension The further the temperature increases we can. The surface tension is the reversible work per unit area needed to elastically stretch/compress a preexisting surface. Increasing the temperature increases the kinetic energy of the molecules. As the molecules get agitated, they. As temperature decreases, surface tension increases. Thus, surface tension forces play a role in its floating behaviour, and the resulting. Effects Of Surface Tension.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Effects Of Surface Tension This effect results from the difference between the potential energy. Let’s consider the effects of these four conditions on surface tension: Increasing the temperature increases the kinetic energy of the molecules. As the molecules get agitated, they. As temperature decreases, surface tension increase s. The surface tension is the reversible work per unit area needed to elastically stretch/compress a preexisting. Effects Of Surface Tension.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and Effects Of Surface Tension Increasing the temperature increases the kinetic energy of the molecules. As the molecules get agitated, they. Thus, surface tension forces play a role in its floating behaviour, and the resulting value of h changes depending on whether surface tension is considered. Let’s consider the effects of these four conditions on surface tension: The surface tension is the reversible work per. Effects Of Surface Tension.
From www.slideserve.com
PPT Biomedical importance of surface tension & Viscosity PowerPoint Effects Of Surface Tension Increasing the temperature increases the kinetic energy of the molecules. The surface tension is the reversible work per unit area needed to elastically stretch/compress a preexisting surface. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Thus, surface. Effects Of Surface Tension.
From www.researchgate.net
Effect of variation of surface tension with temperature when there is Effects Of Surface Tension Let’s consider the effects of these four conditions on surface tension: Increasing the temperature increases the kinetic energy of the molecules. As the molecules get agitated, they. The further the temperature increases we can. Why does surface tension decrease with increasing temperature? The surface tension is the reversible work per unit area needed to elastically stretch/compress a preexisting surface. This. Effects Of Surface Tension.
From www.vrogue.co
Surface Tension Definition Units Epic Examples Effect vrogue.co Effects Of Surface Tension Let’s consider the effects of these four conditions on surface tension: Why does surface tension decrease with increasing temperature? The surface tension is the reversible work per unit area needed to elastically stretch/compress a preexisting surface. Thus, surface tension forces play a role in its floating behaviour, and the resulting value of h changes depending on whether surface tension is. Effects Of Surface Tension.
From www.slideserve.com
PPT Biomedical importance of surface tension PowerPoint Presentation Effects Of Surface Tension Let’s consider the effects of these four conditions on surface tension: Let’s consider the effects of these four conditions on surface tension: As temperature decreases, surface tension increases. The surface tension is the reversible work per unit area needed to elastically stretch/compress a preexisting surface. As temperature decreases, surface tension increase s. As the molecules get agitated, they. Increasing the. Effects Of Surface Tension.
From www.slideserve.com
PPT Physical Pharmacy SURFACE TENSION PowerPoint Presentation, free Effects Of Surface Tension As temperature decreases, surface tension increases. Let’s consider the effects of these four conditions on surface tension: As the molecules get agitated, they. This effect results from the difference between the potential energy. Let’s consider the effects of these four conditions on surface tension: The further the temperature increases we can. The surface tension is the reversible work per unit. Effects Of Surface Tension.