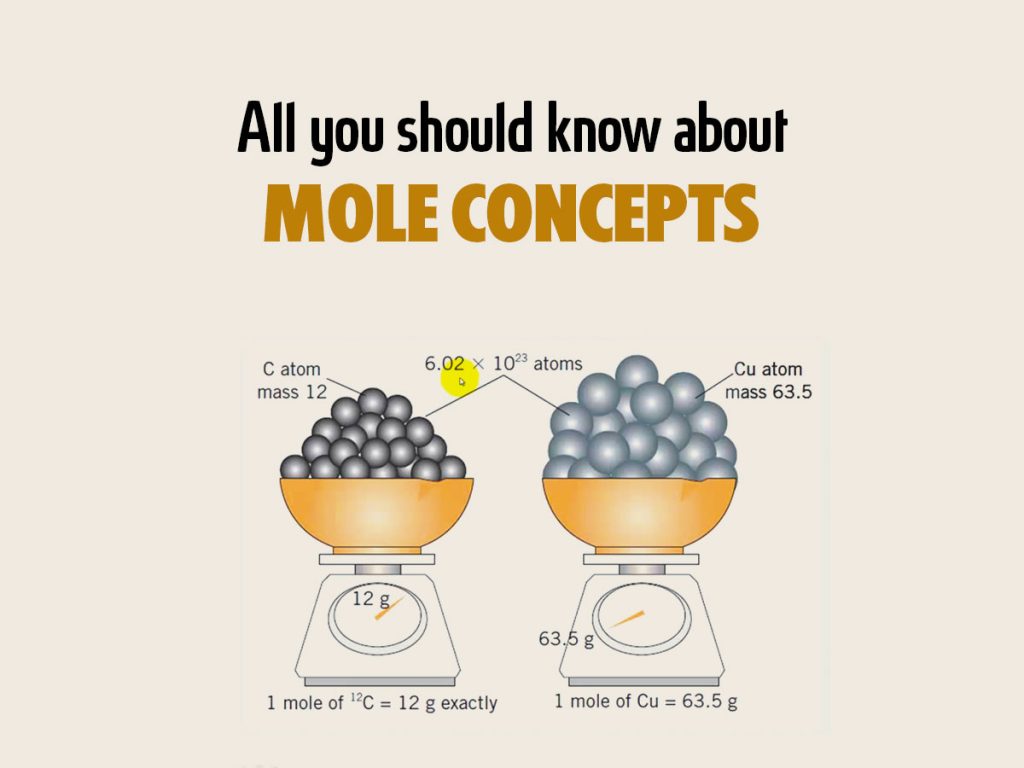
What Does A Mole Measure In Chemistry . The mole is an si unit used to measure the amount of any substance. The mole is the unit of measurement in the international system of units (si) for amount of substance. The abbreviation for mole is mol. One mole is exactly 6.02214076×10 23 particles. The term “mole” is defined as the quantity or mass of a substance that. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. It is defined as the amount of a. A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and pressure contained the.
from periodictable.me
The mole is an si unit used to measure the amount of any substance. The mole is the unit of measurement in the international system of units (si) for amount of substance. The abbreviation for mole is mol. A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. It is defined as the amount of a. The term “mole” is defined as the quantity or mass of a substance that. The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and pressure contained the. One mole is exactly 6.02214076×10 23 particles. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,.
Introduction to the Mole in Chemistry A Brief Explanation
What Does A Mole Measure In Chemistry The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and pressure contained the. The term “mole” is defined as the quantity or mass of a substance that. The abbreviation for mole is mol. It is defined as the amount of a. The mole is an si unit used to measure the amount of any substance. The mole is the unit of measurement in the international system of units (si) for amount of substance. The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and pressure contained the. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. One mole is exactly 6.02214076×10 23 particles.
From www.pinterest.co.uk
What does a mole look like? Teaching chemistry, Chemistry classroom What Does A Mole Measure In Chemistry The mole is the unit of measurement in the international system of units (si) for amount of substance. A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. The term “mole” is defined as the quantity or mass of a substance that. It is defined as the amount of a.. What Does A Mole Measure In Chemistry.
From www.youtube.com
1.2 The mole concept YouTube What Does A Mole Measure In Chemistry The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and pressure contained the. The abbreviation for mole is mol. The mole is the unit of measurement in the international system of units (si) for amount of substance. It is defined as the amount of a. The mole is an si. What Does A Mole Measure In Chemistry.
From ar.inspiredpencil.com
Mole Chemistry What Does A Mole Measure In Chemistry A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. The abbreviation for mole is mol. It is defined as the amount of a. The mole is an si unit used to measure the amount of any substance. The mole is the unit of measurement in the international system of. What Does A Mole Measure In Chemistry.
From facts.net
18 Facts About The Mole In Chemistry What Does A Mole Measure In Chemistry The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and pressure contained the. The mole is an si unit used to measure the amount of any substance. The abbreviation for mole is mol. The mole is the unit of measurement in the international system of units (si) for amount of. What Does A Mole Measure In Chemistry.
From www.amybrownscience.com
Amy Brown Science Chemistry Lab How Big Is A Mole? What Does A Mole Measure In Chemistry The term “mole” is defined as the quantity or mass of a substance that. The mole is an si unit used to measure the amount of any substance. One mole is exactly 6.02214076×10 23 particles. The mole is the unit of measurement in the international system of units (si) for amount of substance. It is defined as the amount of. What Does A Mole Measure In Chemistry.
From www.youtube.com
What is a Mole and How to Use the Mole in Chemistry YouTube What Does A Mole Measure In Chemistry Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and pressure contained the. The mole is the unit of measurement in the international system of units (si) for amount of substance.. What Does A Mole Measure In Chemistry.
From www.slideserve.com
PPT Chemistry 20 Mole Conversions PowerPoint Presentation, free What Does A Mole Measure In Chemistry A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. It is defined as the amount of a. The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and pressure contained the. Mole, standard unit (6.02214076 x 10^23) in chemistry for. What Does A Mole Measure In Chemistry.
From slideplayer.com
What is a mole? Mole Conversion Problems ppt download What Does A Mole Measure In Chemistry One mole is exactly 6.02214076×10 23 particles. The mole is an si unit used to measure the amount of any substance. The mole is the unit of measurement in the international system of units (si) for amount of substance. The term “mole” is defined as the quantity or mass of a substance that. Mole, standard unit (6.02214076 x 10^23) in. What Does A Mole Measure In Chemistry.
From periodictable.me
Introduction to the Mole in Chemistry A Brief Explanation What Does A Mole Measure In Chemistry One mole is exactly 6.02214076×10 23 particles. It is defined as the amount of a. The abbreviation for mole is mol. A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,.. What Does A Mole Measure In Chemistry.
From sciencenotes.org
What Is a Mole In Chemistry? Definition What Does A Mole Measure In Chemistry The abbreviation for mole is mol. The mole is an si unit used to measure the amount of any substance. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. The mole is the unit of measurement in the international system of units (si) for amount of substance. One mole is. What Does A Mole Measure In Chemistry.
From www.pinterest.com
116 best Chemistry Moles, Stoichiometry, and Limiting Reagent images What Does A Mole Measure In Chemistry The abbreviation for mole is mol. It is defined as the amount of a. The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and pressure contained the. The term “mole” is defined as the quantity or mass of a substance that. One mole is exactly 6.02214076×10 23 particles. Mole, standard. What Does A Mole Measure In Chemistry.
From www.coursehero.com
[Solved] A chemist determined by measurements that 0.0400 moles of What Does A Mole Measure In Chemistry A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. The term “mole” is defined as the quantity or mass of a substance that. The abbreviation for mole is mol. The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and. What Does A Mole Measure In Chemistry.
From www.slideserve.com
PPT The Mole A Measurement of Matter PowerPoint Presentation, free What Does A Mole Measure In Chemistry The mole is an si unit used to measure the amount of any substance. It is defined as the amount of a. One mole is exactly 6.02214076×10 23 particles. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. The mole is the unit of measurement in the international system of. What Does A Mole Measure In Chemistry.
From general.chemistrysteps.com
How To Convert Grams To Moles Chemistry Steps What Does A Mole Measure In Chemistry The mole is the unit of measurement in the international system of units (si) for amount of substance. The term “mole” is defined as the quantity or mass of a substance that. The mole is an si unit used to measure the amount of any substance. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very. What Does A Mole Measure In Chemistry.
From ar.inspiredpencil.com
Chemistry Conversion Chart Moles To Grams What Does A Mole Measure In Chemistry The abbreviation for mole is mol. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. The mole is an si unit used to measure the amount of any substance. It is defined as the amount of a. The mole is the unit of measurement in the international system of units. What Does A Mole Measure In Chemistry.
From jesusapa1968.blogspot.com
Jesusa Pa How To Find Mass Of One Mole In Chemistry What Does A Mole Measure In Chemistry A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. One mole is exactly 6.02214076×10 23 particles. The term “mole” is defined as the quantity or mass of a substance that. It is defined as the amount of a. The concept of a mole is based on avogadro’s hypothesis (equal. What Does A Mole Measure In Chemistry.
From www.youtube.com
Understanding Moles (GCSE Chemistry) YouTube What Does A Mole Measure In Chemistry A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. The term “mole” is defined as the quantity or mass of a substance that. The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and pressure contained the. Mole, standard unit. What Does A Mole Measure In Chemistry.
From www.youtube.com
What is a Chemistry Mole....Explained! YouTube What Does A Mole Measure In Chemistry Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. One mole is exactly 6.02214076×10 23 particles. It is defined as the amount of a. The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and pressure contained the. The abbreviation for. What Does A Mole Measure In Chemistry.
From kristinmoonscience.com
What is the Mole? Kristin Moon Science What Does A Mole Measure In Chemistry A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. The mole is the unit of measurement in the international system of units (si) for amount of substance. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. The term “mole”. What Does A Mole Measure In Chemistry.
From www.slideserve.com
PPT Section 7.1 The Mole A Measurement of Matter PowerPoint What Does A Mole Measure In Chemistry The mole is an si unit used to measure the amount of any substance. The abbreviation for mole is mol. The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and pressure contained the. One mole is exactly 6.02214076×10 23 particles. The mole is the unit of measurement in the international. What Does A Mole Measure In Chemistry.
From www.thoughtco.com
What Is a Mole in Chemistry? What Does A Mole Measure In Chemistry One mole is exactly 6.02214076×10 23 particles. A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. The mole is the unit of measurement in the international system of units (si). What Does A Mole Measure In Chemistry.
From www.pinterest.com
A mole conversion is when the mole is able the measure both mass and What Does A Mole Measure In Chemistry It is defined as the amount of a. The mole is the unit of measurement in the international system of units (si) for amount of substance. The mole is an si unit used to measure the amount of any substance. The term “mole” is defined as the quantity or mass of a substance that. The concept of a mole is. What Does A Mole Measure In Chemistry.
From mungfali.com
The Mole Chemistry What Does A Mole Measure In Chemistry The mole is the unit of measurement in the international system of units (si) for amount of substance. The abbreviation for mole is mol. The mole is an si unit used to measure the amount of any substance. The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and pressure contained. What Does A Mole Measure In Chemistry.
From www.slideserve.com
PPT Chemistry 20 Mole Conversions PowerPoint Presentation, free What Does A Mole Measure In Chemistry The term “mole” is defined as the quantity or mass of a substance that. The mole is an si unit used to measure the amount of any substance. It is defined as the amount of a. The abbreviation for mole is mol. The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same. What Does A Mole Measure In Chemistry.
From www.slideshare.net
Measuring moles What Does A Mole Measure In Chemistry A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. The term “mole” is defined as the quantity or mass of a substance that. One mole is exactly 6.02214076×10 23 particles. It is defined as the amount of a. The abbreviation for mole is mol. The mole is the unit. What Does A Mole Measure In Chemistry.
From www.slideshare.net
Measuring moles What Does A Mole Measure In Chemistry Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. The abbreviation for mole is mol. It is defined as the amount of a. The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and pressure contained the. The mole is the. What Does A Mole Measure In Chemistry.
From courses.lumenlearning.com
Formula Mass and the Mole Concept Chemistry for Majors What Does A Mole Measure In Chemistry The abbreviation for mole is mol. It is defined as the amount of a. The mole is the unit of measurement in the international system of units (si) for amount of substance. The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and pressure contained the. The mole is an si. What Does A Mole Measure In Chemistry.
From slideplayer.com
The Mole. ppt download What Does A Mole Measure In Chemistry The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and pressure contained the. The abbreviation for mole is mol. The term “mole” is defined as the quantity or mass of a substance that. The mole is an si unit used to measure the amount of any substance. Mole, standard unit. What Does A Mole Measure In Chemistry.
From slideplayer.com
Lesson 5 Meet the Mole. ppt download What Does A Mole Measure In Chemistry One mole is exactly 6.02214076×10 23 particles. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. The abbreviation for mole is mol. The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and pressure contained the. The term “mole” is defined. What Does A Mole Measure In Chemistry.
From www.slideserve.com
PPT The Mole Concept PowerPoint Presentation, free download ID4787514 What Does A Mole Measure In Chemistry The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and pressure contained the. It is defined as the amount of a. The abbreviation for mole is mol. A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. Mole, standard unit. What Does A Mole Measure In Chemistry.
From slidetodoc.com
THE MOLE Measuring Matter What is a mole What Does A Mole Measure In Chemistry It is defined as the amount of a. One mole is exactly 6.02214076×10 23 particles. A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. The mole is an si unit used to measure the amount of any substance. The concept of a mole is based on avogadro’s hypothesis (equal. What Does A Mole Measure In Chemistry.
From centraltutors.co.uk
SQA Higher Chemistry Mole Calculations involving solutions Central What Does A Mole Measure In Chemistry One mole is exactly 6.02214076×10 23 particles. It is defined as the amount of a. The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and pressure contained the. The mole is an si unit used to measure the amount of any substance. The term “mole” is defined as the quantity. What Does A Mole Measure In Chemistry.
From www.youtube.com
How To Convert Between Moles, Atoms, and Grams In Chemistry QUICK What Does A Mole Measure In Chemistry The abbreviation for mole is mol. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and pressure contained the. One mole is exactly 6.02214076×10 23 particles. The term “mole” is defined. What Does A Mole Measure In Chemistry.
From biobeat.nigms.nih.gov
In Other Words The Measure of a Mole Biomedical Beat Blog National What Does A Mole Measure In Chemistry One mole is exactly 6.02214076×10 23 particles. The term “mole” is defined as the quantity or mass of a substance that. It is defined as the amount of a. The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and pressure contained the. Mole, standard unit (6.02214076 x 10^23) in chemistry. What Does A Mole Measure In Chemistry.
From sciencenotes.org
Mole Fraction Formula and Calculation What Does A Mole Measure In Chemistry It is defined as the amount of a. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. The concept of a mole is based on avogadro’s hypothesis (equal volumes of all gases at the same temperature and pressure contained the. One mole is exactly 6.02214076×10 23 particles. A mole is. What Does A Mole Measure In Chemistry.