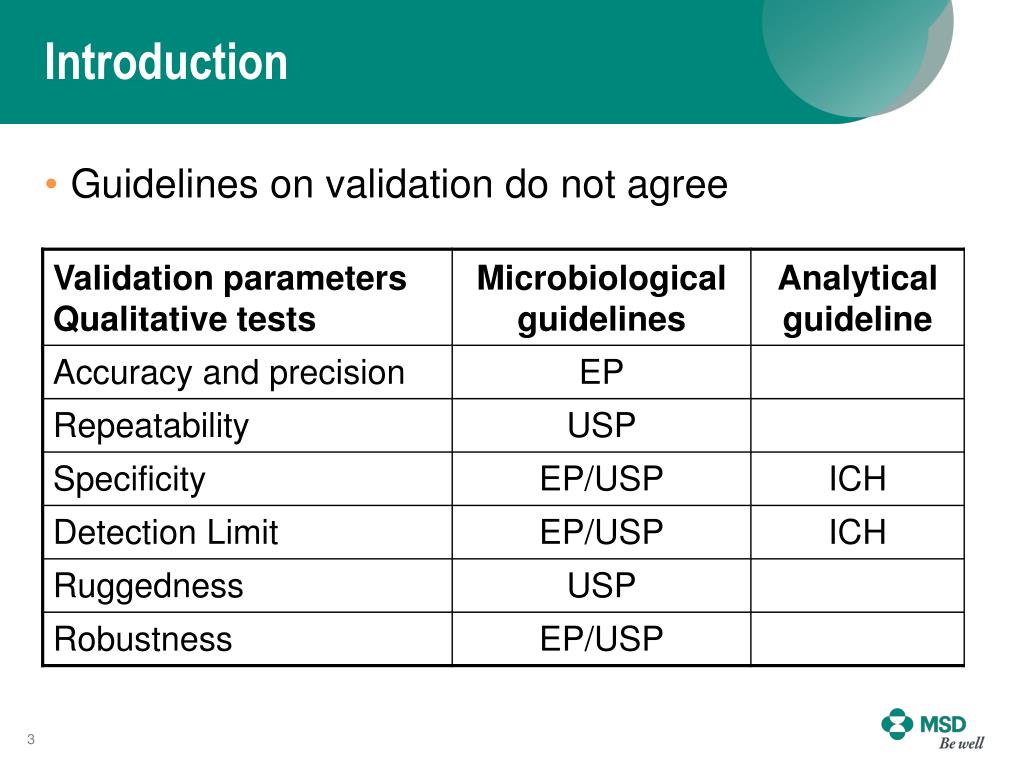
Test Method Validation Ppt . A specific intended use are fulfilled’. ‘the confirmation by examination and the provision of objective evidence that the particular requirements for. This document discusses guidelines for analytical method validation. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. This document discusses guidelines for analytical method validation. It outlines types of analytical methods that. 2 method validation validation of analytical procedures is the process of determining the suitability of a given methodology for providing. This document outlines the key steps in method validation for clinical chemistry laboratories. If there is a linear relationship, test results should be evaluated by appropriate statistical methods, for example, by calculation of a regression line. Validation is important for analytical methods used in pharmaceutical analysis. It defines method validation and.
from www.slideserve.com
Validation is important for analytical methods used in pharmaceutical analysis. It outlines types of analytical methods that. 2 method validation validation of analytical procedures is the process of determining the suitability of a given methodology for providing. If there is a linear relationship, test results should be evaluated by appropriate statistical methods, for example, by calculation of a regression line. This document discusses guidelines for analytical method validation. ‘the confirmation by examination and the provision of objective evidence that the particular requirements for. A specific intended use are fulfilled’. This document outlines the key steps in method validation for clinical chemistry laboratories. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. It defines method validation and.
PPT Validation of Qualitative Microbiological Test Methods PowerPoint
Test Method Validation Ppt It outlines types of analytical methods that. ‘the confirmation by examination and the provision of objective evidence that the particular requirements for. If there is a linear relationship, test results should be evaluated by appropriate statistical methods, for example, by calculation of a regression line. It defines method validation and. Validation is important for analytical methods used in pharmaceutical analysis. This document discusses guidelines for analytical method validation. This document outlines the key steps in method validation for clinical chemistry laboratories. A specific intended use are fulfilled’. It outlines types of analytical methods that. This document discusses guidelines for analytical method validation. 2 method validation validation of analytical procedures is the process of determining the suitability of a given methodology for providing. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in.
From www.slideserve.com
PPT Validation of Qualitative Microbiological Test Methods PowerPoint Test Method Validation Ppt This document discusses guidelines for analytical method validation. 2 method validation validation of analytical procedures is the process of determining the suitability of a given methodology for providing. A specific intended use are fulfilled’. Validation is important for analytical methods used in pharmaceutical analysis. If there is a linear relationship, test results should be evaluated by appropriate statistical methods, for. Test Method Validation Ppt.
From www.slideserve.com
PPT Analytical Methods What, When and How to Validate PowerPoint Test Method Validation Ppt This document outlines the key steps in method validation for clinical chemistry laboratories. ‘the confirmation by examination and the provision of objective evidence that the particular requirements for. This document discusses guidelines for analytical method validation. 2 method validation validation of analytical procedures is the process of determining the suitability of a given methodology for providing. A specific intended use. Test Method Validation Ppt.
From www.slideserve.com
PPT A StepbyStep Guide for Method Validation PowerPoint Test Method Validation Ppt This document outlines the key steps in method validation for clinical chemistry laboratories. It outlines types of analytical methods that. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. This document discusses guidelines for analytical method validation. ‘the confirmation by examination and the provision of objective evidence that the. Test Method Validation Ppt.
From www.slideserve.com
PPT Validation of Analytical Methods PowerPoint Presentation, free Test Method Validation Ppt This document discusses guidelines for analytical method validation. It defines method validation and. A specific intended use are fulfilled’. If there is a linear relationship, test results should be evaluated by appropriate statistical methods, for example, by calculation of a regression line. Validation is important for analytical methods used in pharmaceutical analysis. It outlines types of analytical methods that. This. Test Method Validation Ppt.
From www.collidu.com
Verification and Validation PowerPoint Presentation Slides PPT Template Test Method Validation Ppt This document outlines the key steps in method validation for clinical chemistry laboratories. Validation is important for analytical methods used in pharmaceutical analysis. ‘the confirmation by examination and the provision of objective evidence that the particular requirements for. It defines method validation and. This document discusses guidelines for analytical method validation. If there is a linear relationship, test results should. Test Method Validation Ppt.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online Test Method Validation Ppt This document discusses guidelines for analytical method validation. It outlines types of analytical methods that. If there is a linear relationship, test results should be evaluated by appropriate statistical methods, for example, by calculation of a regression line. Validation is important for analytical methods used in pharmaceutical analysis. 2 method validation validation of analytical procedures is the process of determining. Test Method Validation Ppt.
From www.slideserve.com
PPT Method Validation PowerPoint Presentation, free download ID6087791 Test Method Validation Ppt Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. ‘the confirmation by examination and the provision of objective evidence that the particular requirements for. This document outlines the key steps in method validation for clinical chemistry laboratories. Validation is important for analytical methods used in pharmaceutical analysis. A specific. Test Method Validation Ppt.
From www.slideteam.net
Testing validation process ppt powerpoint presentation professional Test Method Validation Ppt Validation is important for analytical methods used in pharmaceutical analysis. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. 2 method validation validation of analytical procedures is the process of determining the suitability of a given methodology for providing. A specific intended use are fulfilled’. This document discusses guidelines. Test Method Validation Ppt.
From mungfali.com
Test Method Validation Test Method Validation Ppt This document discusses guidelines for analytical method validation. A specific intended use are fulfilled’. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. It defines method validation and. Validation is important for analytical methods used in pharmaceutical analysis. ‘the confirmation by examination and the provision of objective evidence that. Test Method Validation Ppt.
From www.slideserve.com
PPT ANALYTICAL METHOD VALIDATION PowerPoint Presentation, free Test Method Validation Ppt Validation is important for analytical methods used in pharmaceutical analysis. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. This document outlines the key steps in method validation for clinical chemistry laboratories. If there is a linear relationship, test results should be evaluated by appropriate statistical methods, for example,. Test Method Validation Ppt.
From www.slideserve.com
PPT Validation PowerPoint Presentation, free download ID855470 Test Method Validation Ppt It outlines types of analytical methods that. If there is a linear relationship, test results should be evaluated by appropriate statistical methods, for example, by calculation of a regression line. 2 method validation validation of analytical procedures is the process of determining the suitability of a given methodology for providing. Validation is important for analytical methods used in pharmaceutical analysis.. Test Method Validation Ppt.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online Test Method Validation Ppt Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. This document outlines the key steps in method validation for clinical chemistry laboratories. If there is a linear relationship, test results should be evaluated by appropriate statistical methods, for example, by calculation of a regression line. It outlines types of. Test Method Validation Ppt.
From www.slideserve.com
PPT INSTRUMENT AND TEST METHOD VALIDATION PowerPoint Presentation Test Method Validation Ppt A specific intended use are fulfilled’. It defines method validation and. If there is a linear relationship, test results should be evaluated by appropriate statistical methods, for example, by calculation of a regression line. Validation is important for analytical methods used in pharmaceutical analysis. It outlines types of analytical methods that. This document outlines the key steps in method validation. Test Method Validation Ppt.
From ppt-online.org
Method Validation and Verification Protocols for Test Methods Test Method Validation Ppt ‘the confirmation by examination and the provision of objective evidence that the particular requirements for. It outlines types of analytical methods that. If there is a linear relationship, test results should be evaluated by appropriate statistical methods, for example, by calculation of a regression line. This document discusses guidelines for analytical method validation. This document outlines the key steps in. Test Method Validation Ppt.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online Test Method Validation Ppt ‘the confirmation by examination and the provision of objective evidence that the particular requirements for. This document outlines the key steps in method validation for clinical chemistry laboratories. It outlines types of analytical methods that. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. If there is a linear. Test Method Validation Ppt.
From www.slideshare.net
Clinical labmethod validation.ppt Test Method Validation Ppt 2 method validation validation of analytical procedures is the process of determining the suitability of a given methodology for providing. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. If there is a linear relationship, test results should be evaluated by appropriate statistical methods, for example, by calculation of. Test Method Validation Ppt.
From www.slideserve.com
PPT Validation of Qualitative Microbiological Test Methods PowerPoint Test Method Validation Ppt This document discusses guidelines for analytical method validation. This document discusses guidelines for analytical method validation. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. This document outlines the key steps in method validation for clinical chemistry laboratories. 2 method validation validation of analytical procedures is the process of. Test Method Validation Ppt.
From www.slideteam.net
Test Method Validation Protocol Ppt Powerpoint Presentation Show Test Method Validation Ppt A specific intended use are fulfilled’. If there is a linear relationship, test results should be evaluated by appropriate statistical methods, for example, by calculation of a regression line. It defines method validation and. 2 method validation validation of analytical procedures is the process of determining the suitability of a given methodology for providing. Validation is important for analytical methods. Test Method Validation Ppt.
From mungfali.com
Test Method Validation Test Method Validation Ppt ‘the confirmation by examination and the provision of objective evidence that the particular requirements for. 2 method validation validation of analytical procedures is the process of determining the suitability of a given methodology for providing. Validation is important for analytical methods used in pharmaceutical analysis. It defines method validation and. This document discusses guidelines for analytical method validation. This document. Test Method Validation Ppt.
From www.slideteam.net
Test Method Validation Ppt Powerpoint Presentation Model Show Cpb Test Method Validation Ppt Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. It defines method validation and. This document outlines the key steps in method validation for clinical chemistry laboratories. A specific intended use are fulfilled’. If there is a linear relationship, test results should be evaluated by appropriate statistical methods, for. Test Method Validation Ppt.
From www.slideserve.com
PPT Test Method Validation & Verification PowerPoint Presentation Test Method Validation Ppt A specific intended use are fulfilled’. Validation is important for analytical methods used in pharmaceutical analysis. It outlines types of analytical methods that. This document discusses guidelines for analytical method validation. It defines method validation and. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. ‘the confirmation by examination. Test Method Validation Ppt.
From ppt-online.org
Method Validation and Verification Protocols for Test Methods Test Method Validation Ppt It outlines types of analytical methods that. 2 method validation validation of analytical procedures is the process of determining the suitability of a given methodology for providing. This document discusses guidelines for analytical method validation. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. This document discusses guidelines for. Test Method Validation Ppt.
From www.slideserve.com
PPT Test Method Validation & Verification PowerPoint Presentation Test Method Validation Ppt If there is a linear relationship, test results should be evaluated by appropriate statistical methods, for example, by calculation of a regression line. 2 method validation validation of analytical procedures is the process of determining the suitability of a given methodology for providing. This document discusses guidelines for analytical method validation. Validation is important for analytical methods used in pharmaceutical. Test Method Validation Ppt.
From www.collidu.com
Process Validation PowerPoint Presentation Slides PPT Template Test Method Validation Ppt A specific intended use are fulfilled’. Validation is important for analytical methods used in pharmaceutical analysis. This document discusses guidelines for analytical method validation. ‘the confirmation by examination and the provision of objective evidence that the particular requirements for. This document discusses guidelines for analytical method validation. It outlines types of analytical methods that. It defines method validation and. This. Test Method Validation Ppt.
From www.slideserve.com
PPT VALIDATION METHODOLOGY PowerPoint Presentation, free download Test Method Validation Ppt A specific intended use are fulfilled’. 2 method validation validation of analytical procedures is the process of determining the suitability of a given methodology for providing. If there is a linear relationship, test results should be evaluated by appropriate statistical methods, for example, by calculation of a regression line. ‘the confirmation by examination and the provision of objective evidence that. Test Method Validation Ppt.
From www.slideserve.com
PPT ANALYTICAL METHOD VALIDATION PowerPoint Presentation, free Test Method Validation Ppt A specific intended use are fulfilled’. It outlines types of analytical methods that. ‘the confirmation by examination and the provision of objective evidence that the particular requirements for. If there is a linear relationship, test results should be evaluated by appropriate statistical methods, for example, by calculation of a regression line. Laboratories are required to perform analytical validation or verification. Test Method Validation Ppt.
From www.slideserve.com
PPT Validation of Analytical Methods PowerPoint Presentation, free Test Method Validation Ppt Validation is important for analytical methods used in pharmaceutical analysis. A specific intended use are fulfilled’. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. This document outlines the key steps in method validation for clinical chemistry laboratories. It outlines types of analytical methods that. This document discusses guidelines. Test Method Validation Ppt.
From www.slideserve.com
PPT ANALYTICAL METHOD VALIDATION PowerPoint Presentation, free Test Method Validation Ppt It defines method validation and. ‘the confirmation by examination and the provision of objective evidence that the particular requirements for. 2 method validation validation of analytical procedures is the process of determining the suitability of a given methodology for providing. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in.. Test Method Validation Ppt.
From www.slideserve.com
PPT Validation PowerPoint Presentation, free download ID225398 Test Method Validation Ppt 2 method validation validation of analytical procedures is the process of determining the suitability of a given methodology for providing. A specific intended use are fulfilled’. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. It defines method validation and. If there is a linear relationship, test results should. Test Method Validation Ppt.
From www.slideserve.com
PPT Analytical Methods What, When and How to Validate PowerPoint Test Method Validation Ppt This document discusses guidelines for analytical method validation. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. A specific intended use are fulfilled’. Validation is important for analytical methods used in pharmaceutical analysis. ‘the confirmation by examination and the provision of objective evidence that the particular requirements for. It. Test Method Validation Ppt.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online Test Method Validation Ppt It outlines types of analytical methods that. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. ‘the confirmation by examination and the provision of objective evidence that the particular requirements for. 2 method validation validation of analytical procedures is the process of determining the suitability of a given methodology. Test Method Validation Ppt.
From www.slideserve.com
PPT ANALYTICAL METHOD VALIDATION PowerPoint Presentation, free Test Method Validation Ppt ‘the confirmation by examination and the provision of objective evidence that the particular requirements for. A specific intended use are fulfilled’. This document discusses guidelines for analytical method validation. 2 method validation validation of analytical procedures is the process of determining the suitability of a given methodology for providing. This document outlines the key steps in method validation for clinical. Test Method Validation Ppt.
From www.slideteam.net
Testing And Validation Powerpoint Ppt Template Bundles PPT Template Test Method Validation Ppt Validation is important for analytical methods used in pharmaceutical analysis. This document discusses guidelines for analytical method validation. It defines method validation and. 2 method validation validation of analytical procedures is the process of determining the suitability of a given methodology for providing. This document discusses guidelines for analytical method validation. Laboratories are required to perform analytical validation or verification. Test Method Validation Ppt.
From mungfali.com
Test Method Validation Test Method Validation Ppt If there is a linear relationship, test results should be evaluated by appropriate statistical methods, for example, by calculation of a regression line. This document discusses guidelines for analytical method validation. This document outlines the key steps in method validation for clinical chemistry laboratories. A specific intended use are fulfilled’. Validation is important for analytical methods used in pharmaceutical analysis.. Test Method Validation Ppt.
From www.slideserve.com
PPT INSTRUMENT AND TEST METHOD VALIDATION PowerPoint Presentation Test Method Validation Ppt A specific intended use are fulfilled’. If there is a linear relationship, test results should be evaluated by appropriate statistical methods, for example, by calculation of a regression line. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. It defines method validation and. This document discusses guidelines for analytical. Test Method Validation Ppt.