Standard Reduction Potential Iodine . Values are from the following sources: 45 rows standard electrode potentials in aqueous solution at 25°c. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Since the definition of cell. The reference state for amalgams is an infinitely dilute solution of the element in hg. Reduction reactions in acidic solution are written using h + in place of h 3 o +. You may rewrite a reaction by replacing h +. The following table provides e o for selected reduction reactions. Use standard reduction potentials to determine the better. The temperature coefficient, de /dt allows us to calculate the standard potential, e (t), at. Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction.
from www.doubtnut.com
Reduction reactions in acidic solution are written using h + in place of h 3 o +. 45 rows standard electrode potentials in aqueous solution at 25°c. The following table provides e o for selected reduction reactions. Use standard reduction potentials to determine the better. The temperature coefficient, de /dt allows us to calculate the standard potential, e (t), at. Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction. You may rewrite a reaction by replacing h +. The reference state for amalgams is an infinitely dilute solution of the element in hg. Values are from the following sources: Since the definition of cell.
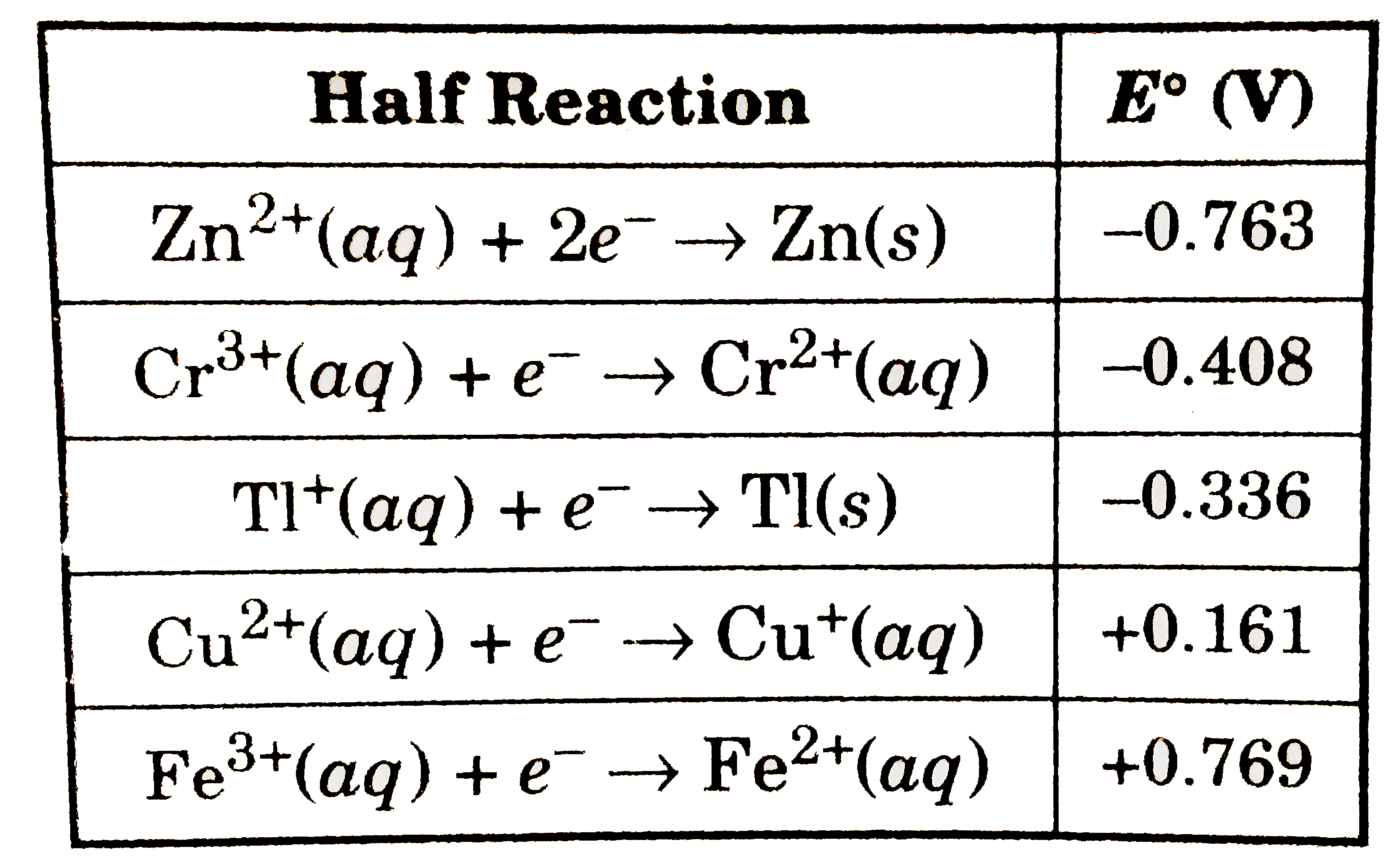
consider the standard reduction potentials (in volts) as shown in figu
Standard Reduction Potential Iodine The following table provides e o for selected reduction reactions. Since the definition of cell. 45 rows standard electrode potentials in aqueous solution at 25°c. Use standard reduction potentials to determine the better. The following table provides e o for selected reduction reactions. Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction. The temperature coefficient, de /dt allows us to calculate the standard potential, e (t), at. Values are from the following sources: The reference state for amalgams is an infinitely dilute solution of the element in hg. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Reduction reactions in acidic solution are written using h + in place of h 3 o +. You may rewrite a reaction by replacing h +.
From www.slideserve.com
PPT Electrochemistry V PowerPoint Presentation, free download ID4246738 Standard Reduction Potential Iodine The following table provides e o for selected reduction reactions. Since the definition of cell. The temperature coefficient, de /dt allows us to calculate the standard potential, e (t), at. 45 rows standard electrode potentials in aqueous solution at 25°c. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she),. Standard Reduction Potential Iodine.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free download ID5480021 Standard Reduction Potential Iodine Values are from the following sources: Reduction reactions in acidic solution are written using h + in place of h 3 o +. Since the definition of cell. Use standard reduction potentials to determine the better. The following table provides e o for selected reduction reactions. The temperature coefficient, de /dt allows us to calculate the standard potential, e (t),. Standard Reduction Potential Iodine.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free download ID5480021 Standard Reduction Potential Iodine The reference state for amalgams is an infinitely dilute solution of the element in hg. You may rewrite a reaction by replacing h +. The following table provides e o for selected reduction reactions. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Having compared many reactions to. Standard Reduction Potential Iodine.
From askfilo.com
The standard reduction potentials of several ions are as follows S.No.lo.. Standard Reduction Potential Iodine Reduction reactions in acidic solution are written using h + in place of h 3 o +. Since the definition of cell. The temperature coefficient, de /dt allows us to calculate the standard potential, e (t), at. 45 rows standard electrode potentials in aqueous solution at 25°c. Values are from the following sources: You may rewrite a reaction by replacing. Standard Reduction Potential Iodine.
From www.slideserve.com
PPT 21 Electrochemistry PowerPoint Presentation, free download ID826545 Standard Reduction Potential Iodine You may rewrite a reaction by replacing h +. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Values are from the following sources: Reduction reactions in acidic solution are written using h + in place of h 3 o +. The reference state for amalgams is an. Standard Reduction Potential Iodine.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free download ID5699060 Standard Reduction Potential Iodine Values are from the following sources: The following table provides e o for selected reduction reactions. You may rewrite a reaction by replacing h +. The temperature coefficient, de /dt allows us to calculate the standard potential, e (t), at. 45 rows standard electrode potentials in aqueous solution at 25°c. 372 rows the data below tabulates standard electrode potentials (e. Standard Reduction Potential Iodine.
From users.highland.edu
Standard Potentials Standard Reduction Potential Iodine Values are from the following sources: Since the definition of cell. The reference state for amalgams is an infinitely dilute solution of the element in hg. Use standard reduction potentials to determine the better. Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction. 372 rows the data below tabulates standard electrode potentials. Standard Reduction Potential Iodine.
From chem.libretexts.org
Standard Potentials Chemistry LibreTexts Standard Reduction Potential Iodine 45 rows standard electrode potentials in aqueous solution at 25°c. Since the definition of cell. Use standard reduction potentials to determine the better. The reference state for amalgams is an infinitely dilute solution of the element in hg. The temperature coefficient, de /dt allows us to calculate the standard potential, e (t), at. Reduction reactions in acidic solution are written. Standard Reduction Potential Iodine.
From www.slideserve.com
PPT ELECTROCHEMISTRY PowerPoint Presentation, free download ID3222616 Standard Reduction Potential Iodine Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction. 45 rows standard electrode potentials in aqueous solution at 25°c. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The following table provides e o for selected reduction reactions. The reference. Standard Reduction Potential Iodine.
From saylordotorg.github.io
Standard Potentials Standard Reduction Potential Iodine Values are from the following sources: Since the definition of cell. The following table provides e o for selected reduction reactions. Reduction reactions in acidic solution are written using h + in place of h 3 o +. The reference state for amalgams is an infinitely dilute solution of the element in hg. Use standard reduction potentials to determine the. Standard Reduction Potential Iodine.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID541675 Standard Reduction Potential Iodine Reduction reactions in acidic solution are written using h + in place of h 3 o +. The following table provides e o for selected reduction reactions. Use standard reduction potentials to determine the better. Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction. Values are from the following sources: 45 rows. Standard Reduction Potential Iodine.
From www.chegg.com
Solved Use the table below to answer the following Standard Reduction Potential Iodine Use standard reduction potentials to determine the better. Reduction reactions in acidic solution are written using h + in place of h 3 o +. You may rewrite a reaction by replacing h +. Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction. Values are from the following sources: Since the definition. Standard Reduction Potential Iodine.
From pveducation.org
Standard Potential PVEducation Standard Reduction Potential Iodine The reference state for amalgams is an infinitely dilute solution of the element in hg. Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction. Values are from the following sources: You may rewrite a reaction by replacing h +. The temperature coefficient, de /dt allows us to calculate the standard potential, e. Standard Reduction Potential Iodine.
From www.chegg.com
Solved Table 1 Some standard reduction potentials (298 K) Standard Reduction Potential Iodine Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The following table provides e o for selected reduction reactions. The temperature coefficient, de /dt allows us to calculate the standard potential,. Standard Reduction Potential Iodine.
From www.chem.fsu.edu
Lab Purpose Standard Reduction Potential Iodine Values are from the following sources: 45 rows standard electrode potentials in aqueous solution at 25°c. You may rewrite a reaction by replacing h +. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The temperature coefficient, de /dt allows us to calculate the standard potential, e (t),. Standard Reduction Potential Iodine.
From www.slideserve.com
PPT Chapter 18 Electrochemistry PowerPoint Presentation, free download ID5410403 Standard Reduction Potential Iodine Use standard reduction potentials to determine the better. The reference state for amalgams is an infinitely dilute solution of the element in hg. The following table provides e o for selected reduction reactions. You may rewrite a reaction by replacing h +. Values are from the following sources: The temperature coefficient, de /dt allows us to calculate the standard potential,. Standard Reduction Potential Iodine.
From www.chegg.com
Solved What is the standard reduction potential of Y3+ given Standard Reduction Potential Iodine 45 rows standard electrode potentials in aqueous solution at 25°c. Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The following table provides e o for selected reduction reactions. Use standard. Standard Reduction Potential Iodine.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Iodine The following table provides e o for selected reduction reactions. Since the definition of cell. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. 45 rows standard electrode potentials in aqueous solution at 25°c. The reference state for amalgams is an infinitely dilute solution of the element in. Standard Reduction Potential Iodine.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials Chemistry Standard Reduction Potential Iodine 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Reduction reactions in acidic solution are written using h + in place of h 3 o +. The following table provides e o for selected reduction reactions. 45 rows standard electrode potentials in aqueous solution at 25°c. The reference. Standard Reduction Potential Iodine.
From protonstalk.com
Electrochemical Series Features, Applications, Examples ProtonsTalk Standard Reduction Potential Iodine 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. You may rewrite a reaction by replacing h +. 45 rows standard electrode potentials in aqueous solution at 25°c. Use standard reduction potentials to determine the better. Since the definition of cell. The temperature coefficient, de /dt allows us. Standard Reduction Potential Iodine.
From www.slideserve.com
PPT Chemistry and Electricity PowerPoint Presentation, free download ID258546 Standard Reduction Potential Iodine The temperature coefficient, de /dt allows us to calculate the standard potential, e (t), at. Since the definition of cell. Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction. Values are from the following sources: The following table provides e o for selected reduction reactions. 45 rows standard electrode potentials in aqueous. Standard Reduction Potential Iodine.
From www.docsity.com
Standard Reduction Potential Metabolic Biochemistry BIBC 102 Docsity Standard Reduction Potential Iodine Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction. The temperature coefficient, de /dt allows us to calculate the standard potential, e (t), at. You may rewrite a reaction by replacing h +. The reference state for amalgams is an infinitely dilute solution of the element in hg. Use standard reduction potentials. Standard Reduction Potential Iodine.
From www.slideserve.com
PPT Oxidation and Reduction PowerPoint Presentation, free download ID4653489 Standard Reduction Potential Iodine 45 rows standard electrode potentials in aqueous solution at 25°c. The following table provides e o for selected reduction reactions. Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction. The reference state for amalgams is an infinitely dilute solution of the element in hg. You may rewrite a reaction by replacing h. Standard Reduction Potential Iodine.
From www.slideserve.com
PPT Electrochemistry & Virus Templated Electrodes PowerPoint Presentation ID3067840 Standard Reduction Potential Iodine Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction. The temperature coefficient, de /dt allows us to calculate the standard potential, e (t), at. You may rewrite a reaction by replacing h +. 45 rows standard electrode potentials in aqueous solution at 25°c. Use standard reduction potentials to determine the better. Reduction. Standard Reduction Potential Iodine.
From www.pinterest.co.kr
pe=log[e] measuved in volts.if pe is negative reduced, if pe is positive It is oxidized. The Standard Reduction Potential Iodine Values are from the following sources: 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction. 45 rows standard electrode potentials in aqueous solution at 25°c. Use standard reduction potentials to determine. Standard Reduction Potential Iodine.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Iodine Since the definition of cell. 45 rows standard electrode potentials in aqueous solution at 25°c. The following table provides e o for selected reduction reactions. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. You may rewrite a reaction by replacing h +. Values are from the following. Standard Reduction Potential Iodine.
From www.doubtnut.com
consider the standard reduction potentials (in volts) as shown in figu Standard Reduction Potential Iodine The temperature coefficient, de /dt allows us to calculate the standard potential, e (t), at. 45 rows standard electrode potentials in aqueous solution at 25°c. Use standard reduction potentials to determine the better. The reference state for amalgams is an infinitely dilute solution of the element in hg. Reduction reactions in acidic solution are written using h + in place. Standard Reduction Potential Iodine.
From www.solutioninn.com
[Solved] Use the table of standard reduction poten SolutionInn Standard Reduction Potential Iodine 45 rows standard electrode potentials in aqueous solution at 25°c. Use standard reduction potentials to determine the better. The reference state for amalgams is an infinitely dilute solution of the element in hg. You may rewrite a reaction by replacing h +. Values are from the following sources: 372 rows the data below tabulates standard electrode potentials (e °), in. Standard Reduction Potential Iodine.
From www.numerade.com
SOLVED Knowing the following standard reduction potentials I2 + 2e → 2I E° = +0.535 V Ca2 Standard Reduction Potential Iodine Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction. The following table provides e o for selected reduction reactions. Use standard reduction potentials to determine the better. You may rewrite a reaction by replacing h +. Since the definition of cell. Values are from the following sources: The reference state for amalgams. Standard Reduction Potential Iodine.
From www.flinnsci.com
Standard Reduction Potential Chart Flinn Scientific Standard Reduction Potential Iodine You may rewrite a reaction by replacing h +. Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The reference state for amalgams is an infinitely dilute solution of the element. Standard Reduction Potential Iodine.
From www.slideserve.com
PPT ELECTROCHEMISTRY Chapter 21 PowerPoint Presentation, free download ID812189 Standard Reduction Potential Iodine The temperature coefficient, de /dt allows us to calculate the standard potential, e (t), at. Use standard reduction potentials to determine the better. Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction. The following table provides e o for selected reduction reactions. Reduction reactions in acidic solution are written using h +. Standard Reduction Potential Iodine.
From www.researchgate.net
Standard iodine reduction potentials in seawater. In basic aqueous... Download Scientific Diagram Standard Reduction Potential Iodine The temperature coefficient, de /dt allows us to calculate the standard potential, e (t), at. Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction. 45 rows standard electrode potentials in aqueous solution at 25°c. The following table provides e o for selected reduction reactions. Reduction reactions in acidic solution are written using. Standard Reduction Potential Iodine.
From www.youtube.com
The diagonal rule of the standard reduction potential table (Part II) YouTube Standard Reduction Potential Iodine 45 rows standard electrode potentials in aqueous solution at 25°c. Values are from the following sources: Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction. You may rewrite a reaction by replacing h +. The reference state for amalgams is an infinitely dilute solution of the element in hg. Use standard reduction. Standard Reduction Potential Iodine.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Reduction Potential Iodine 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. You may rewrite a reaction by replacing h +. The reference state for amalgams is an infinitely dilute solution of the element in hg. Values are from the following sources: 45 rows standard electrode potentials in aqueous solution at. Standard Reduction Potential Iodine.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Reduction Potential Iodine 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Values are from the following sources: Having compared many reactions to the standard hydrogen potential, we can now make a table of reduction. The reference state for amalgams is an infinitely dilute solution of the element in hg. Reduction. Standard Reduction Potential Iodine.