Gmp Guidelines For Tablet Manufacturing . The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Guidelines on good manufacturing practice specific to. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on the quality of. Specific gmp requirements relevant to classes of products such as sterile pharmaceuticals or biological medicinal products are provided in a. Good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in their production processes.
from flairpharma.com
Specific gmp requirements relevant to classes of products such as sterile pharmaceuticals or biological medicinal products are provided in a. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on the quality of. Good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in their production processes. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on. Guidelines on good manufacturing practice specific to.
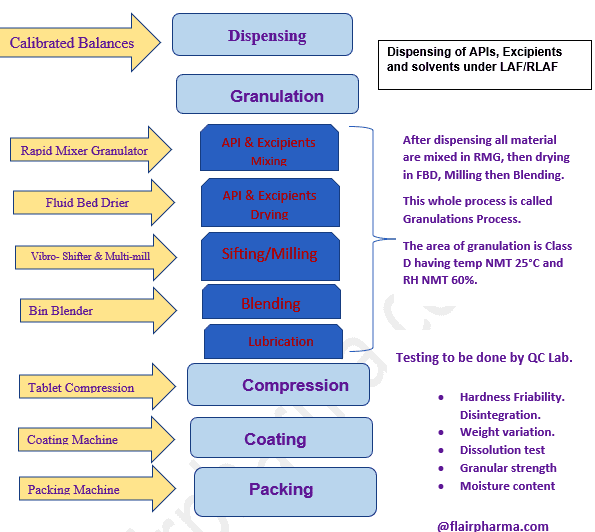
Manufacturing Process Of Oral Solid Doses 22 » Flair Pharma The
Gmp Guidelines For Tablet Manufacturing This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on the quality of. Good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in their production processes. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Specific gmp requirements relevant to classes of products such as sterile pharmaceuticals or biological medicinal products are provided in a. Guidelines on good manufacturing practice specific to.
From www.slideshare.net
Back To Basic Gmp Gmp Guidelines For Tablet Manufacturing Guidelines on good manufacturing practice specific to. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on. Specific gmp requirements relevant to classes of products such as sterile pharmaceuticals or biological medicinal products are provided in a. This guide to gmp shall be used. Gmp Guidelines For Tablet Manufacturing.
From imagetou.com
Tablet Manufacturing Process Flow Chart Image to u Gmp Guidelines For Tablet Manufacturing This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on the quality of. Good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in their production processes. This guide to gmp shall be used as a standard to justify gmp. Gmp Guidelines For Tablet Manufacturing.
From www.studypool.com
SOLUTION Tablet manufacturing process Studypool Gmp Guidelines For Tablet Manufacturing This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Guidelines on good manufacturing practice specific to. Good manufacturing practice (gmp) describes the minimum standard that. Gmp Guidelines For Tablet Manufacturing.
From www.studypool.com
SOLUTION Tablet manufacturing process Studypool Gmp Guidelines For Tablet Manufacturing This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on the quality of. Guidelines on good manufacturing practice specific to. Good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in their production processes. Specific gmp requirements relevant to classes. Gmp Guidelines For Tablet Manufacturing.
From www.studocu.com
Tablet manufacturing manual 2023 School of Pharmacy and Biomedical Gmp Guidelines For Tablet Manufacturing This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on the quality of. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on. Good manufacturing practice (gmp) describes. Gmp Guidelines For Tablet Manufacturing.
From www.gmp7.com
GMP, Good Manufacturing Practice, SOP Quality Documents for FDA EU Gmp Guidelines For Tablet Manufacturing The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Specific gmp requirements relevant to classes of products such as sterile pharmaceuticals or biological medicinal products are provided in a. Guidelines on good manufacturing practice specific to. This guide to gmp shall be used as a standard to justify gmp status, which. Gmp Guidelines For Tablet Manufacturing.
From pharmajia.com
Granulation Process In Tablet Manufacturing A Comprehensive Approach Gmp Guidelines For Tablet Manufacturing Good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in their production processes. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Specific gmp requirements relevant to classes of products such as sterile pharmaceuticals or biological medicinal products are provided in a. This guide to gmp. Gmp Guidelines For Tablet Manufacturing.
From flairpharma.com
Manufacturing Process Of Oral Solid Doses 22 » Flair Pharma The Gmp Guidelines For Tablet Manufacturing This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on the quality of. Guidelines on good manufacturing practice specific to. Specific gmp requirements relevant to classes of products such as sterile pharmaceuticals or biological medicinal products are provided in a. This guide to gmp. Gmp Guidelines For Tablet Manufacturing.
From ivenpharmatech.en.made-in-china.com
Turnkey Oral Tablet Manufacturing Plant/ Complete Solid Dose Processing Gmp Guidelines For Tablet Manufacturing Good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in their production processes. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on the quality of. Guidelines on good manufacturing practice specific to. The european medicines agency's (ema) provides. Gmp Guidelines For Tablet Manufacturing.
From www.allfordrugs.com
GMP All About Drugs Gmp Guidelines For Tablet Manufacturing This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on the quality of. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on. The european medicines agency's (ema). Gmp Guidelines For Tablet Manufacturing.
From instantgmp.com
Integrated Quality Management System GMP Compliance InstantGMP Gmp Guidelines For Tablet Manufacturing This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on the quality of. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on. Good manufacturing practice (gmp) describes. Gmp Guidelines For Tablet Manufacturing.
From www.youtube.com
TABLET MANUFACTURING PROCESS GRANULATION DIRECT COMPRESSION YouTube Gmp Guidelines For Tablet Manufacturing The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Specific gmp requirements relevant to classes of products such as sterile pharmaceuticals or biological medicinal products are provided in a. Good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in their production processes. This guide to gmp. Gmp Guidelines For Tablet Manufacturing.
From mavink.com
Tablet Manufacturing Process Flow Chart Gmp Guidelines For Tablet Manufacturing Specific gmp requirements relevant to classes of products such as sterile pharmaceuticals or biological medicinal products are provided in a. Good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in their production processes. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the. Gmp Guidelines For Tablet Manufacturing.
From www.pharmaguideline.com
Tablet Manufacturing Process An Overview Pharmaguideline Gmp Guidelines For Tablet Manufacturing The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on the quality of. Guidelines on good manufacturing practice specific to. Good manufacturing practice (gmp) describes the. Gmp Guidelines For Tablet Manufacturing.
From www.youtube.com
Tablet Manufacturing Process and EquipmentsTablet Granulation Gmp Guidelines For Tablet Manufacturing Good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in their production processes. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Specific gmp requirements relevant to classes of products such as sterile pharmaceuticals or biological medicinal products are provided in a. Guidelines on good manufacturing. Gmp Guidelines For Tablet Manufacturing.
From know-how-academy.teachable.com
Tablet Manufacturing Techniques KnowHow Academy Gmp Guidelines For Tablet Manufacturing This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on the quality of. Good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in their production processes. Specific gmp requirements relevant to classes of products such as sterile pharmaceuticals or. Gmp Guidelines For Tablet Manufacturing.
From www.slideserve.com
PPT Tablet manufacturing process 322 PHT PowerPoint Presentation Gmp Guidelines For Tablet Manufacturing Specific gmp requirements relevant to classes of products such as sterile pharmaceuticals or biological medicinal products are provided in a. Guidelines on good manufacturing practice specific to. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on. Good manufacturing practice (gmp) describes the minimum. Gmp Guidelines For Tablet Manufacturing.
From www.researchgate.net
Process flow diagram which highlights various steps during tablet Gmp Guidelines For Tablet Manufacturing Specific gmp requirements relevant to classes of products such as sterile pharmaceuticals or biological medicinal products are provided in a. Good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in their production processes. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. This guide to gmp. Gmp Guidelines For Tablet Manufacturing.
From www.inmedpharma.com
What are the GMP manufacturing standards for different products? Gmp Guidelines For Tablet Manufacturing This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Specific gmp requirements relevant to classes of products such as sterile pharmaceuticals or biological medicinal products. Gmp Guidelines For Tablet Manufacturing.
From www.pharmapproach.com
Manufacture of Tablets by Dry granulation method Gmp Guidelines For Tablet Manufacturing Guidelines on good manufacturing practice specific to. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Specific gmp requirements relevant to classes of products such. Gmp Guidelines For Tablet Manufacturing.
From 1streporting.com
The GMP Audit Checklist Pharmaceutical Industry Should Be Using Gmp Guidelines For Tablet Manufacturing This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on the quality of. The european medicines agency's (ema). Gmp Guidelines For Tablet Manufacturing.
From mavink.com
Tablet Manufacturing Process Flow Chart Gmp Guidelines For Tablet Manufacturing This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on the quality of. Good manufacturing practice (gmp) describes. Gmp Guidelines For Tablet Manufacturing.
From www.slideshare.net
Layout of Tablet Manufacturing Section Gmp Guidelines For Tablet Manufacturing This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on the quality of. Specific gmp requirements relevant to classes of products such as sterile pharmaceuticals or biological medicinal products are provided in a. Guidelines on good manufacturing practice specific to. Good manufacturing practice (gmp). Gmp Guidelines For Tablet Manufacturing.
From www.studypool.com
SOLUTION Tablet manufacturing process Studypool Gmp Guidelines For Tablet Manufacturing The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in their production processes. Specific gmp requirements relevant to classes of products such as sterile pharmaceuticals or biological medicinal products are provided in a. Guidelines on good manufacturing. Gmp Guidelines For Tablet Manufacturing.
From www.regdesk.co
FDA Guidance on Manufacturing Site Changes Supplements Submission Gmp Guidelines For Tablet Manufacturing This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on. Guidelines on good manufacturing practice specific to. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on the. Gmp Guidelines For Tablet Manufacturing.
From smpnutra.com
The Dietary Tablet Manufacturing Process Everything To Know Gmp Guidelines For Tablet Manufacturing Good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in their production processes. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on the quality of. The european medicines agency's (ema) provides answers to frequently asked questions on good. Gmp Guidelines For Tablet Manufacturing.
From www.pharmaguideline.com
Tablet Manufacturing Process Flowchart Pharmaguideline Gmp Guidelines For Tablet Manufacturing The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in their production processes. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification. Gmp Guidelines For Tablet Manufacturing.
From www.youtube.com
Good Manufacturing Practices GMP in Pharmaceuticals YouTube Gmp Guidelines For Tablet Manufacturing Specific gmp requirements relevant to classes of products such as sterile pharmaceuticals or biological medicinal products are provided in a. Good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in their production processes. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Guidelines on good manufacturing. Gmp Guidelines For Tablet Manufacturing.
From www.youtube.com
Tablet Manufacturing Process Granulation Compression L5 Unit2 Gmp Guidelines For Tablet Manufacturing The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Specific gmp requirements relevant to classes of products such as sterile pharmaceuticals or biological medicinal products are provided in a. Good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in their production processes. This guide to gmp. Gmp Guidelines For Tablet Manufacturing.
From www.youtube.com
Tablet Manufacturing Explained Different Stages of Tablet Gmp Guidelines For Tablet Manufacturing Good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in their production processes. Guidelines on good manufacturing practice specific to. Specific gmp requirements relevant to classes of products such as sterile pharmaceuticals or biological medicinal products are provided in a. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice. Gmp Guidelines For Tablet Manufacturing.
From bceweb.org
Tablet Manufacturing Process Flow Chart A Visual Reference of Charts Gmp Guidelines For Tablet Manufacturing This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on the quality of. Specific gmp requirements relevant to. Gmp Guidelines For Tablet Manufacturing.
From www.scribd.com
GMP Guide for Drug Products Quality Management System Risk Management Gmp Guidelines For Tablet Manufacturing Good manufacturing practice (gmp) describes the minimum standard that a medicines manufacturer must meet in their production processes. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on. This guide to gmp shall be used as a standard to justify gmp status, which constitutes. Gmp Guidelines For Tablet Manufacturing.
From www.studypool.com
SOLUTION Pharmacetics 1 tablet manufacturing ppt Studypool Gmp Guidelines For Tablet Manufacturing This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on the quality of. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Specific gmp requirements relevant to classes of products such as sterile pharmaceuticals or. Gmp Guidelines For Tablet Manufacturing.
From smpnutra.com
Who Gives GMP Certification? Premier Manufacturing Gmp Guidelines For Tablet Manufacturing This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on. Guidelines on good manufacturing practice specific to. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the who certification scheme on the. Gmp Guidelines For Tablet Manufacturing.
From www.studypool.com
SOLUTION Tablet manufacturing dry granulation method short notes Gmp Guidelines For Tablet Manufacturing The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good. Specific gmp requirements relevant to classes of products such as sterile pharmaceuticals or biological medicinal products are provided in a. This guide to gmp shall be used as a standard to justify gmp status, which constitutes one of the elements of the. Gmp Guidelines For Tablet Manufacturing.