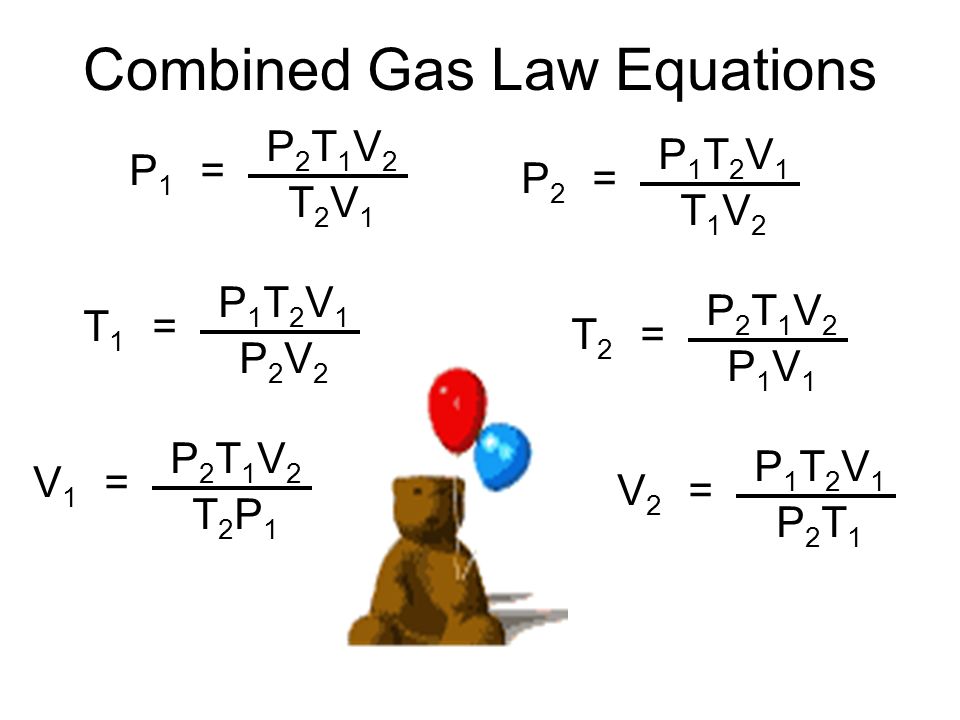
Combined Gas Law Finding Volume . the combined gas law is obtained by rearranging the ideal gas law, from pv = nrt. the combined gas law relates pressure, temperature, and volume when everything else is held constant (mainly the moles of. it is important to recognize the p total is the 98.0 value. P total is the combined pressure of the dry gas and the water vapor. the combined gas law expresses the relationship between the pressure, volume, and absolute. in this module, the relationship between pressure, temperature, volume, and amount of a gas are described and how. the combined gas law relates the variables pressure, temperature, and volume whereas the ideal gas law relates these three. The following pages introduces the law of combined gases. From this, we obtain an expression for the r: = \;\frac { { {\rm. what happens to the volume of gases when the react?
from inspiritvr.com
the combined gas law expresses the relationship between the pressure, volume, and absolute. P total is the combined pressure of the dry gas and the water vapor. in this module, the relationship between pressure, temperature, volume, and amount of a gas are described and how. it is important to recognize the p total is the 98.0 value. the combined gas law is obtained by rearranging the ideal gas law, from pv = nrt. From this, we obtain an expression for the r: the combined gas law relates pressure, temperature, and volume when everything else is held constant (mainly the moles of. The following pages introduces the law of combined gases. what happens to the volume of gases when the react? = \;\frac { { {\rm.
Combined Gas Law Study Guide Inspirit Learning Inc
Combined Gas Law Finding Volume it is important to recognize the p total is the 98.0 value. the combined gas law expresses the relationship between the pressure, volume, and absolute. it is important to recognize the p total is the 98.0 value. The following pages introduces the law of combined gases. in this module, the relationship between pressure, temperature, volume, and amount of a gas are described and how. P total is the combined pressure of the dry gas and the water vapor. the combined gas law relates the variables pressure, temperature, and volume whereas the ideal gas law relates these three. From this, we obtain an expression for the r: what happens to the volume of gases when the react? = \;\frac { { {\rm. the combined gas law relates pressure, temperature, and volume when everything else is held constant (mainly the moles of. the combined gas law is obtained by rearranging the ideal gas law, from pv = nrt.
From www.expii.com
Combined Gas Law — Overview & Calculations Expii Combined Gas Law Finding Volume the combined gas law is obtained by rearranging the ideal gas law, from pv = nrt. P total is the combined pressure of the dry gas and the water vapor. the combined gas law expresses the relationship between the pressure, volume, and absolute. = \;\frac { { {\rm. the combined gas law relates the variables pressure, temperature,. Combined Gas Law Finding Volume.
From slidetodoc.com
The Combined Gas Law The Combined Gas Law Combined Gas Law Finding Volume the combined gas law relates the variables pressure, temperature, and volume whereas the ideal gas law relates these three. From this, we obtain an expression for the r: in this module, the relationship between pressure, temperature, volume, and amount of a gas are described and how. The following pages introduces the law of combined gases. the combined. Combined Gas Law Finding Volume.
From www.slideserve.com
PPT Combined Gas Law PowerPoint Presentation, free download ID2654120 Combined Gas Law Finding Volume From this, we obtain an expression for the r: the combined gas law is obtained by rearranging the ideal gas law, from pv = nrt. the combined gas law relates the variables pressure, temperature, and volume whereas the ideal gas law relates these three. = \;\frac { { {\rm. the combined gas law expresses the relationship between. Combined Gas Law Finding Volume.
From www.sliderbase.com
Gas Laws Presentation Chemistry Combined Gas Law Finding Volume From this, we obtain an expression for the r: P total is the combined pressure of the dry gas and the water vapor. the combined gas law relates pressure, temperature, and volume when everything else is held constant (mainly the moles of. the combined gas law is obtained by rearranging the ideal gas law, from pv = nrt.. Combined Gas Law Finding Volume.
From worksheetcalzariniop.z14.web.core.windows.net
Combined Gas Law Description Combined Gas Law Finding Volume = \;\frac { { {\rm. in this module, the relationship between pressure, temperature, volume, and amount of a gas are described and how. the combined gas law expresses the relationship between the pressure, volume, and absolute. the combined gas law is obtained by rearranging the ideal gas law, from pv = nrt. the combined gas law. Combined Gas Law Finding Volume.
From www.youtube.com
maxresdefault.jpg Combined Gas Law Finding Volume in this module, the relationship between pressure, temperature, volume, and amount of a gas are described and how. what happens to the volume of gases when the react? The following pages introduces the law of combined gases. the combined gas law relates pressure, temperature, and volume when everything else is held constant (mainly the moles of. . Combined Gas Law Finding Volume.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID4199554 Combined Gas Law Finding Volume the combined gas law relates pressure, temperature, and volume when everything else is held constant (mainly the moles of. the combined gas law expresses the relationship between the pressure, volume, and absolute. the combined gas law is obtained by rearranging the ideal gas law, from pv = nrt. From this, we obtain an expression for the r:. Combined Gas Law Finding Volume.
From lessonlibraryungirths.z21.web.core.windows.net
Combined Gas Law Explained Combined Gas Law Finding Volume = \;\frac { { {\rm. it is important to recognize the p total is the 98.0 value. P total is the combined pressure of the dry gas and the water vapor. From this, we obtain an expression for the r: what happens to the volume of gases when the react? in this module, the relationship between pressure,. Combined Gas Law Finding Volume.
From worksheetaidansuskm.z13.web.core.windows.net
Combined Gas Law Explained Combined Gas Law Finding Volume the combined gas law relates the variables pressure, temperature, and volume whereas the ideal gas law relates these three. the combined gas law is obtained by rearranging the ideal gas law, from pv = nrt. The following pages introduces the law of combined gases. what happens to the volume of gases when the react? P total is. Combined Gas Law Finding Volume.
From www.slideserve.com
PPT Chapter 8 Gases, Liquids, and Solids PowerPoint Presentation Combined Gas Law Finding Volume P total is the combined pressure of the dry gas and the water vapor. the combined gas law relates the variables pressure, temperature, and volume whereas the ideal gas law relates these three. the combined gas law expresses the relationship between the pressure, volume, and absolute. the combined gas law relates pressure, temperature, and volume when everything. Combined Gas Law Finding Volume.
From materialfullagonises.z13.web.core.windows.net
Combined Gas Law Explained Combined Gas Law Finding Volume P total is the combined pressure of the dry gas and the water vapor. the combined gas law expresses the relationship between the pressure, volume, and absolute. in this module, the relationship between pressure, temperature, volume, and amount of a gas are described and how. the combined gas law is obtained by rearranging the ideal gas law,. Combined Gas Law Finding Volume.
From www.slideserve.com
PPT The General Gas Equation Combined Gas Law PowerPoint Presentation Combined Gas Law Finding Volume = \;\frac { { {\rm. in this module, the relationship between pressure, temperature, volume, and amount of a gas are described and how. the combined gas law relates pressure, temperature, and volume when everything else is held constant (mainly the moles of. From this, we obtain an expression for the r: the combined gas law expresses the. Combined Gas Law Finding Volume.
From www.slideserve.com
PPT Gas!!! PowerPoint Presentation, free download ID6011745 Combined Gas Law Finding Volume in this module, the relationship between pressure, temperature, volume, and amount of a gas are described and how. From this, we obtain an expression for the r: The following pages introduces the law of combined gases. the combined gas law expresses the relationship between the pressure, volume, and absolute. it is important to recognize the p total. Combined Gas Law Finding Volume.
From www.youtube.com
Combined Gas Law Pressure, Volume and Temperature Straight Science Combined Gas Law Finding Volume the combined gas law is obtained by rearranging the ideal gas law, from pv = nrt. = \;\frac { { {\rm. the combined gas law expresses the relationship between the pressure, volume, and absolute. From this, we obtain an expression for the r: what happens to the volume of gases when the react? P total is the. Combined Gas Law Finding Volume.
From www.slideserve.com
PPT The Combined Gas Law PowerPoint Presentation, free download ID Combined Gas Law Finding Volume = \;\frac { { {\rm. P total is the combined pressure of the dry gas and the water vapor. the combined gas law relates the variables pressure, temperature, and volume whereas the ideal gas law relates these three. it is important to recognize the p total is the 98.0 value. what happens to the volume of gases. Combined Gas Law Finding Volume.
From www.studocu.com
Assignment of Combined Gas Laws Combined Gas Laws A gas occupies a Combined Gas Law Finding Volume From this, we obtain an expression for the r: = \;\frac { { {\rm. the combined gas law is obtained by rearranging the ideal gas law, from pv = nrt. in this module, the relationship between pressure, temperature, volume, and amount of a gas are described and how. the combined gas law relates the variables pressure, temperature,. Combined Gas Law Finding Volume.
From www.slideshare.net
Gas laws Diagrams Combined Gas Law Finding Volume The following pages introduces the law of combined gases. the combined gas law relates the variables pressure, temperature, and volume whereas the ideal gas law relates these three. the combined gas law relates pressure, temperature, and volume when everything else is held constant (mainly the moles of. From this, we obtain an expression for the r: in. Combined Gas Law Finding Volume.
From dxottpstg.blob.core.windows.net
How To Use Combined Gas Law at Joan Ray blog Combined Gas Law Finding Volume the combined gas law relates the variables pressure, temperature, and volume whereas the ideal gas law relates these three. what happens to the volume of gases when the react? the combined gas law relates pressure, temperature, and volume when everything else is held constant (mainly the moles of. the combined gas law is obtained by rearranging. Combined Gas Law Finding Volume.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint Combined Gas Law Finding Volume what happens to the volume of gases when the react? the combined gas law relates pressure, temperature, and volume when everything else is held constant (mainly the moles of. P total is the combined pressure of the dry gas and the water vapor. From this, we obtain an expression for the r: the combined gas law is. Combined Gas Law Finding Volume.
From www.slideshare.net
Notes gas laws Combined Gas Law Finding Volume the combined gas law is obtained by rearranging the ideal gas law, from pv = nrt. = \;\frac { { {\rm. in this module, the relationship between pressure, temperature, volume, and amount of a gas are described and how. P total is the combined pressure of the dry gas and the water vapor. From this, we obtain an. Combined Gas Law Finding Volume.
From www.slideserve.com
PPT Gases PowerPoint Presentation, free download ID6019279 Combined Gas Law Finding Volume = \;\frac { { {\rm. in this module, the relationship between pressure, temperature, volume, and amount of a gas are described and how. the combined gas law expresses the relationship between the pressure, volume, and absolute. the combined gas law is obtained by rearranging the ideal gas law, from pv = nrt. P total is the combined. Combined Gas Law Finding Volume.
From sciencenotes.org
Combined Gas Law Definition, Formula, Examples Combined Gas Law Finding Volume it is important to recognize the p total is the 98.0 value. the combined gas law relates pressure, temperature, and volume when everything else is held constant (mainly the moles of. the combined gas law is obtained by rearranging the ideal gas law, from pv = nrt. what happens to the volume of gases when the. Combined Gas Law Finding Volume.
From exotnbeyi.blob.core.windows.net
Combined Gas Law Ppt at Dennis House blog Combined Gas Law Finding Volume the combined gas law relates the variables pressure, temperature, and volume whereas the ideal gas law relates these three. P total is the combined pressure of the dry gas and the water vapor. what happens to the volume of gases when the react? it is important to recognize the p total is the 98.0 value. the. Combined Gas Law Finding Volume.
From studylib.net
The Combined Gas Law Combined Gas Law Finding Volume the combined gas law expresses the relationship between the pressure, volume, and absolute. the combined gas law relates pressure, temperature, and volume when everything else is held constant (mainly the moles of. = \;\frac { { {\rm. From this, we obtain an expression for the r: P total is the combined pressure of the dry gas and the. Combined Gas Law Finding Volume.
From inspiritvr.com
Combined Gas Law Study Guide Inspirit Learning Inc Combined Gas Law Finding Volume the combined gas law expresses the relationship between the pressure, volume, and absolute. P total is the combined pressure of the dry gas and the water vapor. The following pages introduces the law of combined gases. it is important to recognize the p total is the 98.0 value. = \;\frac { { {\rm. the combined gas law. Combined Gas Law Finding Volume.
From www.slideserve.com
PPT Combined Gas Law PowerPoint Presentation, free download ID3252378 Combined Gas Law Finding Volume The following pages introduces the law of combined gases. the combined gas law relates pressure, temperature, and volume when everything else is held constant (mainly the moles of. the combined gas law is obtained by rearranging the ideal gas law, from pv = nrt. what happens to the volume of gases when the react? = \;\frac {. Combined Gas Law Finding Volume.
From drcalef.com
The Combined Gas Law Pressure, Volume, and Temperature Combined Gas Law Finding Volume the combined gas law relates pressure, temperature, and volume when everything else is held constant (mainly the moles of. The following pages introduces the law of combined gases. it is important to recognize the p total is the 98.0 value. what happens to the volume of gases when the react? the combined gas law expresses the. Combined Gas Law Finding Volume.
From slidetodoc.com
The Combined Gas Law The Combined Gas Law Combined Gas Law Finding Volume the combined gas law is obtained by rearranging the ideal gas law, from pv = nrt. P total is the combined pressure of the dry gas and the water vapor. the combined gas law expresses the relationship between the pressure, volume, and absolute. From this, we obtain an expression for the r: = \;\frac { { {\rm. . Combined Gas Law Finding Volume.
From www.slideserve.com
PPT Ch. 13 Notes Gas Laws PowerPoint Presentation ID6765779 Combined Gas Law Finding Volume = \;\frac { { {\rm. The following pages introduces the law of combined gases. P total is the combined pressure of the dry gas and the water vapor. the combined gas law relates pressure, temperature, and volume when everything else is held constant (mainly the moles of. what happens to the volume of gases when the react? . Combined Gas Law Finding Volume.
From www.slideserve.com
PPT Combined Gas Law PowerPoint Presentation, free download ID5864985 Combined Gas Law Finding Volume the combined gas law relates the variables pressure, temperature, and volume whereas the ideal gas law relates these three. P total is the combined pressure of the dry gas and the water vapor. From this, we obtain an expression for the r: it is important to recognize the p total is the 98.0 value. the combined gas. Combined Gas Law Finding Volume.
From slideplayer.com
Combined Gas Law Equation Problems ppt download Combined Gas Law Finding Volume From this, we obtain an expression for the r: P total is the combined pressure of the dry gas and the water vapor. = \;\frac { { {\rm. the combined gas law relates the variables pressure, temperature, and volume whereas the ideal gas law relates these three. it is important to recognize the p total is the 98.0. Combined Gas Law Finding Volume.
From www.slideserve.com
PPT Properties of Gases & Gas Laws PowerPoint Presentation, free Combined Gas Law Finding Volume what happens to the volume of gases when the react? From this, we obtain an expression for the r: = \;\frac { { {\rm. the combined gas law is obtained by rearranging the ideal gas law, from pv = nrt. it is important to recognize the p total is the 98.0 value. P total is the combined. Combined Gas Law Finding Volume.
From www.slideserve.com
PPT Chapter 4 Introduction to Gases PowerPoint Presentation, free Combined Gas Law Finding Volume the combined gas law is obtained by rearranging the ideal gas law, from pv = nrt. From this, we obtain an expression for the r: it is important to recognize the p total is the 98.0 value. what happens to the volume of gases when the react? in this module, the relationship between pressure, temperature, volume,. Combined Gas Law Finding Volume.
From owlcation.com
The Theories and Behavior of Gas Owlcation Combined Gas Law Finding Volume the combined gas law relates pressure, temperature, and volume when everything else is held constant (mainly the moles of. the combined gas law relates the variables pressure, temperature, and volume whereas the ideal gas law relates these three. = \;\frac { { {\rm. it is important to recognize the p total is the 98.0 value. P total. Combined Gas Law Finding Volume.
From www.youtube.com
11.6 The Combined Gas Law Pressure, Volume, & Temperature YouTube Combined Gas Law Finding Volume From this, we obtain an expression for the r: the combined gas law expresses the relationship between the pressure, volume, and absolute. = \;\frac { { {\rm. it is important to recognize the p total is the 98.0 value. The following pages introduces the law of combined gases. in this module, the relationship between pressure, temperature, volume,. Combined Gas Law Finding Volume.