Colour Change Of Kmno4 In Titration . In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is colourless to light. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. Because the added permanganate is rapidly consumed, adding small volumes of a 0.0247 m kmno 4 solution, which has a deep purple color, to the rhubarb extract does not initially change the color. In close proximity to the endpoint, the action of the indicator is. When the colour changes from light pink to deep. The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. In the reaction, kmno4 is reduced to mn+2, changing from a purple color to a colorless solution. Obtain the final volume reading from the calibration scale on the buret. Once all the oxalic acid has been consumed, the solution. It will change colour in the presence of the kmno4 sample.
from www.chegg.com
In close proximity to the endpoint, the action of the indicator is. The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is colourless to light. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. When the colour changes from light pink to deep. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. It will change colour in the presence of the kmno4 sample. Obtain the final volume reading from the calibration scale on the buret. Because the added permanganate is rapidly consumed, adding small volumes of a 0.0247 m kmno 4 solution, which has a deep purple color, to the rhubarb extract does not initially change the color. Once all the oxalic acid has been consumed, the solution.
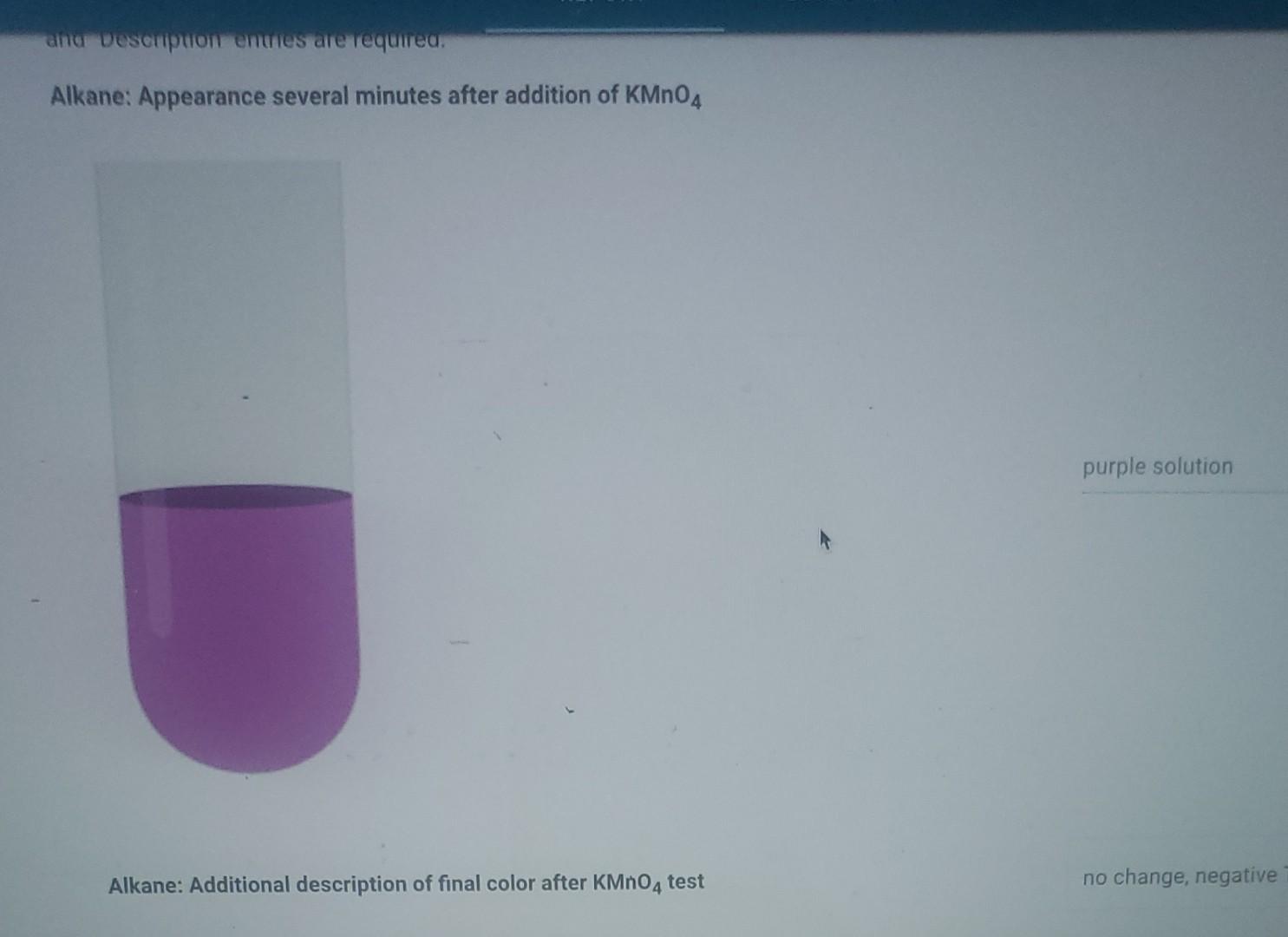
Solved about the color of solution. Virtual KMnO4 Test Key
Colour Change Of Kmno4 In Titration In close proximity to the endpoint, the action of the indicator is. In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is colourless to light. Obtain the final volume reading from the calibration scale on the buret. In close proximity to the endpoint, the action of the indicator is. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. When the colour changes from light pink to deep. In the reaction, kmno4 is reduced to mn+2, changing from a purple color to a colorless solution. It will change colour in the presence of the kmno4 sample. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Because the added permanganate is rapidly consumed, adding small volumes of a 0.0247 m kmno 4 solution, which has a deep purple color, to the rhubarb extract does not initially change the color. Once all the oxalic acid has been consumed, the solution. The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration.
From www.toppr.com
It was observed that in the initial additions of KMnO4 in the titration Colour Change Of Kmno4 In Titration The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. It will change colour in the presence of the kmno4 sample. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. In close proximity to the endpoint, the action. Colour Change Of Kmno4 In Titration.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Colour Change Of Kmno4 In Titration In close proximity to the endpoint, the action of the indicator is. Obtain the final volume reading from the calibration scale on the buret. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. Because the added permanganate is rapidly consumed, adding small volumes of a 0.0247 m kmno 4 solution,. Colour Change Of Kmno4 In Titration.
From www.chegg.com
SET5The color change in the titration of KMnO4 with Colour Change Of Kmno4 In Titration It will change colour in the presence of the kmno4 sample. When the colour changes from light pink to deep. In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is colourless to light. Because the added permanganate is rapidly consumed, adding small volumes of a 0.0247 m kmno 4 solution, which. Colour Change Of Kmno4 In Titration.
From imagetou.com
Color Of Kmno4 Solution Image to u Colour Change Of Kmno4 In Titration The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. Because the added permanganate is rapidly consumed, adding small volumes of a 0.0247 m kmno 4 solution, which has a deep purple color, to the rhubarb extract does not initially change the color. When the colour changes from light pink to deep.. Colour Change Of Kmno4 In Titration.
From www.numerade.com
SOLVED In KMNO4 versus Mohr Salt titration, why does colour changes to Colour Change Of Kmno4 In Titration Because the added permanganate is rapidly consumed, adding small volumes of a 0.0247 m kmno 4 solution, which has a deep purple color, to the rhubarb extract does not initially change the color. In the reaction, kmno4 is reduced to mn+2, changing from a purple color to a colorless solution. In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour. Colour Change Of Kmno4 In Titration.
From www.youtube.com
Determination of Concentration of KMnO4 Soution using Ferrous Ammonium Colour Change Of Kmno4 In Titration The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. When the colour changes from light pink to deep. It will change colour in the presence of the kmno4 sample. In close proximity to the endpoint, the action of the indicator is. Obtain the final volume reading from the calibration scale on. Colour Change Of Kmno4 In Titration.
From fphoto.photoshelter.com
science chemistry titration potassium permanganate Fundamental Colour Change Of Kmno4 In Titration Obtain the final volume reading from the calibration scale on the buret. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. Once all the oxalic acid has been consumed, the solution. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example. Colour Change Of Kmno4 In Titration.
From www.youtube.com
KMNO4 Titration result color. The correct pink color. YouTube Colour Change Of Kmno4 In Titration The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In close proximity to the endpoint, the action of the indicator is. In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is colourless to light. In the reaction, kmno4. Colour Change Of Kmno4 In Titration.
From www.youtube.com
colour change reaction with kmno4 kmno4 chemistry ytshorts YouTube Colour Change Of Kmno4 In Titration It will change colour in the presence of the kmno4 sample. Because the added permanganate is rapidly consumed, adding small volumes of a 0.0247 m kmno 4 solution, which has a deep purple color, to the rhubarb extract does not initially change the color. The pinkish color produced by the first drop of excess kmno4 signals the end point for. Colour Change Of Kmno4 In Titration.
From www.youtube.com
kmno4 colour change from pink to green (mn oxidation state changes+7 to Colour Change Of Kmno4 In Titration Obtain the final volume reading from the calibration scale on the buret. When the colour changes from light pink to deep. It will change colour in the presence of the kmno4 sample. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. The pinkish color produced by the. Colour Change Of Kmno4 In Titration.
From www.youtube.com
KMnO4 Titration Time Lapse [HD] YouTube Colour Change Of Kmno4 In Titration In the reaction, kmno4 is reduced to mn+2, changing from a purple color to a colorless solution. It will change colour in the presence of the kmno4 sample. The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. When the colour changes from light pink to deep. Because the added permanganate is. Colour Change Of Kmno4 In Titration.
From chemistrypubs.com
Titration of Oxalic Acid with KMnO4 Chemistrupubs Colour Change Of Kmno4 In Titration The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Once all the oxalic acid has been consumed, the solution. When the colour changes from light pink to deep. In close proximity to the endpoint, the action of the indicator is. The pinkish color produced by the first. Colour Change Of Kmno4 In Titration.
From www.youtube.com
KMnO4 Redox Titration With FeSO4.7H20 YouTube Colour Change Of Kmno4 In Titration In close proximity to the endpoint, the action of the indicator is. When the colour changes from light pink to deep. Because the added permanganate is rapidly consumed, adding small volumes of a 0.0247 m kmno 4 solution, which has a deep purple color, to the rhubarb extract does not initially change the color. The pinkish color produced by the. Colour Change Of Kmno4 In Titration.
From www.youtube.com
Titration Of Kmno4 Vs Oxalic Acid The Ultimate Guide Titration Colour Change Of Kmno4 In Titration Obtain the final volume reading from the calibration scale on the buret. When the colour changes from light pink to deep. Because the added permanganate is rapidly consumed, adding small volumes of a 0.0247 m kmno 4 solution, which has a deep purple color, to the rhubarb extract does not initially change the color. The titration of potassium permanganate (kmno. Colour Change Of Kmno4 In Titration.
From www.chegg.com
Solved about the color of solution. Virtual KMnO4 Test Key Colour Change Of Kmno4 In Titration An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Once. Colour Change Of Kmno4 In Titration.
From www.youtube.com
KMnO4 Reacts with Citric Acid Awesome Visible Colour Change A Colour Change Of Kmno4 In Titration When the colour changes from light pink to deep. It will change colour in the presence of the kmno4 sample. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox. Colour Change Of Kmno4 In Titration.
From www.youtube.com
Diffusion of KMnO4 Complete YouTube Colour Change Of Kmno4 In Titration Obtain the final volume reading from the calibration scale on the buret. In the reaction, kmno4 is reduced to mn+2, changing from a purple color to a colorless solution. In close proximity to the endpoint, the action of the indicator is. Once all the oxalic acid has been consumed, the solution. Because the added permanganate is rapidly consumed, adding small. Colour Change Of Kmno4 In Titration.
From tuitiontube.com
KMnO4 + H2SO4 + Na2SO3 = K2SO4 + MnSO4 + Na2SO4 + H2O Tuition Tube Colour Change Of Kmno4 In Titration It will change colour in the presence of the kmno4 sample. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. Once all the oxalic acid has been consumed, the solution. The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. Because the. Colour Change Of Kmno4 In Titration.
From www.youtube.com
KMNO4+ Water colour changechemistry Jchemistry YouTube Colour Change Of Kmno4 In Titration When the colour changes from light pink to deep. In the reaction, kmno4 is reduced to mn+2, changing from a purple color to a colorless solution. In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is colourless to light. Because the added permanganate is rapidly consumed, adding small volumes of a. Colour Change Of Kmno4 In Titration.
From slideplayer.com
Titration Colour Changes ppt download Colour Change Of Kmno4 In Titration The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. Obtain the final volume reading from the calibration scale on the buret. It will change colour in the presence of the kmno4 sample. In close proximity to the endpoint, the action of the indicator is. The titration of potassium permanganate (kmno 4). Colour Change Of Kmno4 In Titration.
From www.youtube.com
KMnO4 instant color change reaction YouTube Colour Change Of Kmno4 In Titration An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. In the reaction, kmno4 is reduced to mn+2, changing from a purple color to a colorless solution. Because the added permanganate is rapidly consumed, adding small volumes of a 0.0247 m kmno 4 solution, which has a deep purple color, to. Colour Change Of Kmno4 In Titration.
From loeahtchl.blob.core.windows.net
Color Of Kmno4 Solution at Malcolm Broussard blog Colour Change Of Kmno4 In Titration In close proximity to the endpoint, the action of the indicator is. In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is colourless to light. Once all the oxalic acid has been consumed, the solution. In the reaction, kmno4 is reduced to mn+2, changing from a purple color to a colorless. Colour Change Of Kmno4 In Titration.
From www.youtube.com
The Colour of KMnO4 Why KMnO4 is coloured? why potassium permanganate Colour Change Of Kmno4 In Titration In the reaction, kmno4 is reduced to mn+2, changing from a purple color to a colorless solution. It will change colour in the presence of the kmno4 sample. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. Because the added permanganate is rapidly consumed, adding small volumes of a 0.0247. Colour Change Of Kmno4 In Titration.
From www.youtube.com
Preparation, Standardization of 0.1N KMnO4 solution YouTube Colour Change Of Kmno4 In Titration It will change colour in the presence of the kmno4 sample. Because the added permanganate is rapidly consumed, adding small volumes of a 0.0247 m kmno 4 solution, which has a deep purple color, to the rhubarb extract does not initially change the color. Obtain the final volume reading from the calibration scale on the buret. Once all the oxalic. Colour Change Of Kmno4 In Titration.
From chemistrypubs.com
Titration of Oxalic Acid with KMnO4 Chemistrupubs Colour Change Of Kmno4 In Titration In the reaction, kmno4 is reduced to mn+2, changing from a purple color to a colorless solution. In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is colourless to light. It will change colour in the presence of the kmno4 sample. Once all the oxalic acid has been consumed, the solution.. Colour Change Of Kmno4 In Titration.
From www.youtube.com
Standardization of KMnO4 Redox Titration KMnO4 Vs Fe2+ Mohrs salt Colour Change Of Kmno4 In Titration Obtain the final volume reading from the calibration scale on the buret. In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is colourless to light. When the colour changes from light pink to deep. Because the added permanganate is rapidly consumed, adding small volumes of a 0.0247 m kmno 4 solution,. Colour Change Of Kmno4 In Titration.
From www.toppr.com
64 V 4 X 1 English o Review Later Which of the following reaction not Colour Change Of Kmno4 In Titration Because the added permanganate is rapidly consumed, adding small volumes of a 0.0247 m kmno 4 solution, which has a deep purple color, to the rhubarb extract does not initially change the color. Obtain the final volume reading from the calibration scale on the buret. Once all the oxalic acid has been consumed, the solution. In the reaction, kmno4 is. Colour Change Of Kmno4 In Titration.
From www.researchgate.net
How to reduce the amount of MnO2 generated from titration of a solution Colour Change Of Kmno4 In Titration It will change colour in the presence of the kmno4 sample. In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is colourless to light. When the colour changes from light pink to deep. The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration.. Colour Change Of Kmno4 In Titration.
From www.numerade.com
SOLVED Potassium permanganate (KMnO4) was used as an oxidizing titrant Colour Change Of Kmno4 In Titration When the colour changes from light pink to deep. Because the added permanganate is rapidly consumed, adding small volumes of a 0.0247 m kmno 4 solution, which has a deep purple color, to the rhubarb extract does not initially change the color. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an. Colour Change Of Kmno4 In Titration.
From slideplayer.com
Titration Colour Changes ppt download Colour Change Of Kmno4 In Titration Obtain the final volume reading from the calibration scale on the buret. Once all the oxalic acid has been consumed, the solution. It will change colour in the presence of the kmno4 sample. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Because the added permanganate is. Colour Change Of Kmno4 In Titration.
From www.youtube.com
Titration oxalic acid vs kmno4 class 12 practical readings and Colour Change Of Kmno4 In Titration When the colour changes from light pink to deep. Because the added permanganate is rapidly consumed, adding small volumes of a 0.0247 m kmno 4 solution, which has a deep purple color, to the rhubarb extract does not initially change the color. Once all the oxalic acid has been consumed, the solution. Obtain the final volume reading from the calibration. Colour Change Of Kmno4 In Titration.
From www.youtube.com
Titration of Oxalic acid vs KMnO4B.Sc 1st sem (NEP)Dr.Chhavi Purwar Colour Change Of Kmno4 In Titration In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is colourless to light. In close proximity to the endpoint, the action of the indicator is. It will change colour in the presence of the kmno4 sample. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o. Colour Change Of Kmno4 In Titration.
From slideplayer.com
Titration Colour Changes ppt download Colour Change Of Kmno4 In Titration Because the added permanganate is rapidly consumed, adding small volumes of a 0.0247 m kmno 4 solution, which has a deep purple color, to the rhubarb extract does not initially change the color. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. When the colour changes from. Colour Change Of Kmno4 In Titration.
From www.youtube.com
Changing the colour solution of KMnO4 by adding H2SO4 YouTube Colour Change Of Kmno4 In Titration In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is colourless to light. Obtain the final volume reading from the calibration scale on the buret. In close proximity to the endpoint, the action of the indicator is. The pinkish color produced by the first drop of excess kmno4 signals the end. Colour Change Of Kmno4 In Titration.
From www.coursehero.com
[Solved] titrant =KMnO4. In acidbase titrations indicators are used to Colour Change Of Kmno4 In Titration An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. In close proximity to the endpoint, the action of the indicator is. Because the added permanganate is rapidly consumed, adding small volumes of. Colour Change Of Kmno4 In Titration.