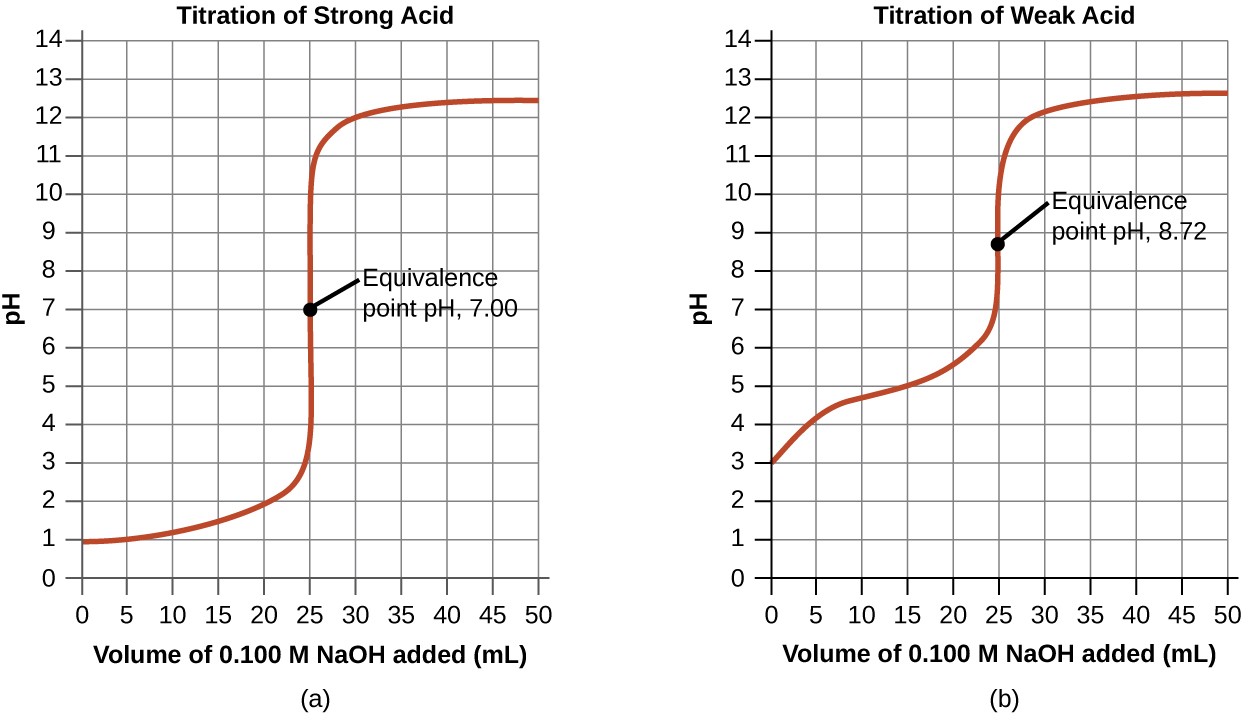
Titration Curve Naoh . A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). The volume of the titrant added. The titration of a weak acid with strong base. It usually only occurs until a ph of around 10. The image of a titration curve of a weak acid with a strong base is seen below. What are ph titration curves. The steep portion of the curve prior to the equivalence point is short. All of the characteristics described above can be seen within it. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution;
from psu.pb.unizin.org
All of the characteristics described above can be seen within it. It usually only occurs until a ph of around 10. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. The image of a titration curve of a weak acid with a strong base is seen below. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). What are ph titration curves. The titration of a weak acid with strong base. The steep portion of the curve prior to the equivalence point is short. The volume of the titrant added.
AcidBase Titrations (14.7) Chemistry 110
Titration Curve Naoh All of the characteristics described above can be seen within it. All of the characteristics described above can be seen within it. The steep portion of the curve prior to the equivalence point is short. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). The image of a titration curve of a weak acid with a strong base is seen below. The volume of the titrant added. The titration of a weak acid with strong base. It usually only occurs until a ph of around 10. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. What are ph titration curves.
From mungfali.com
HCl NaOH Titration Titration Curve Naoh The titration of a weak acid with strong base. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. It usually only occurs until a ph of around 10. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the. Titration Curve Naoh.
From mungfali.com
HCl NaOH Titration Titration Curve Naoh The volume of the titrant added. What are ph titration curves. The steep portion of the curve prior to the equivalence point is short. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. Titration is a technique used in neutralisation reactions between acids and alkalis to. Titration Curve Naoh.
From www.researchgate.net
Titration of 1 mL diluted bath (H2SO4/H3PO4) with 0.5 M NaOH in water Titration Curve Naoh It usually only occurs until a ph of around 10. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. The steep portion of the curve prior to the equivalence point is short. The volume of the titrant added. The titration of a weak acid with strong. Titration Curve Naoh.
From www.learnsci.com
LearnSci Smart Worksheet Determine Concentration of HCl by Titration Titration Curve Naoh The steep portion of the curve prior to the equivalence point is short. The image of a titration curve of a weak acid with a strong base is seen below. The volume of the titrant added. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. The. Titration Curve Naoh.
From www.numerade.com
SOLVED 5. Shown below is the titration curve of glycine (Gly), using Titration Curve Naoh Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. It usually only occurs until a ph of around 10. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; The titration of a weak acid with. Titration Curve Naoh.
From chart-studio.plotly.com
KHP and NaOH Titration Curve line chart made by Kylclk plotly Titration Curve Naoh All of the characteristics described above can be seen within it. The titration of a weak acid with strong base. The image of a titration curve of a weak acid with a strong base is seen below. It usually only occurs until a ph of around 10. The volume of the titrant added. The steep portion of the curve prior. Titration Curve Naoh.
From www.chemistryscl.com
NaOH and HCl Titration Curves Selecting Indicators Titration Curve Naoh Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. The volume of the titrant added. A titration is carried out for 25.00 ml of 0.100 m. Titration Curve Naoh.
From www.chegg.com
Solved Titration of 50.0mL of 0.100M H3PO4 with 0.100M NaOH Titration Curve Naoh A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). The steep portion of the curve prior to the equivalence point is short. The image of a titration curve of a weak acid with a strong base is seen below.. Titration Curve Naoh.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Curve Naoh All of the characteristics described above can be seen within it. The volume of the titrant added. The steep portion of the curve prior to the equivalence point is short. The image of a titration curve of a weak acid with a strong base is seen below. Titration is a technique used in neutralisation reactions between acids and alkalis to. Titration Curve Naoh.
From www.researchgate.net
Titration curve of 0.001 mol L −1 1 with 0.1 mol L −1 NaOH in 96 Titration Curve Naoh The steep portion of the curve prior to the equivalence point is short. The titration of a weak acid with strong base. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). It usually only occurs until a ph of. Titration Curve Naoh.
From moodle.tau.ac.il
AcidBase Titration Curves Titration Curve Naoh The image of a titration curve of a weak acid with a strong base is seen below. The titration of a weak acid with strong base. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). It usually only occurs. Titration Curve Naoh.
From www.numerade.com
SOLVED 5. (4 pts) Shown below is the titration curve of the amino acid Titration Curve Naoh A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). The steep portion of the curve prior to the equivalence point is short. The titration of a weak acid with strong base. What are ph titration curves. Titrations are often. Titration Curve Naoh.
From byjus.com
The graph of pH during the titration of NaOH and HCl Titration Curve Naoh Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. The steep portion of the curve prior to the equivalence point is short. The titration of a weak acid with strong base. It usually only occurs until a ph of around 10. Titration is a technique used. Titration Curve Naoh.
From chart-studio.plotly.com
Acetic Acid (CH3COOH) + Sodium Hydroxide (NaOH) Titration scatter Titration Curve Naoh Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; The image of a titration curve of a weak acid with a strong base is seen below. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the.. Titration Curve Naoh.
From www.youtube.com
DAT Titration Curve of Weak Acid Strong Base (CH3COOH and NaOH Titration Curve Naoh All of the characteristics described above can be seen within it. The image of a titration curve of a weak acid with a strong base is seen below. The steep portion of the curve prior to the equivalence point is short. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown. Titration Curve Naoh.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Titration Curve Naoh What are ph titration curves. The steep portion of the curve prior to the equivalence point is short. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; The titration of a weak acid with strong base. The image of a titration curve of a weak acid with a strong. Titration Curve Naoh.
From www.solutioninn.com
[Solved] The student performs a second titration u SolutionInn Titration Curve Naoh The volume of the titrant added. The steep portion of the curve prior to the equivalence point is short. All of the characteristics described above can be seen within it. It usually only occurs until a ph of around 10. The titration of a weak acid with strong base. A titration is carried out for 25.00 ml of 0.100 m. Titration Curve Naoh.
From mungfali.com
HCl NaOH Titration Titration Curve Naoh Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. The titration of a weak acid with strong base. It usually only occurs until a ph of around 10. The image of a titration curve of a weak acid with a strong base is seen below. The. Titration Curve Naoh.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Curve Naoh The image of a titration curve of a weak acid with a strong base is seen below. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; The steep portion of the curve prior to the equivalence point is short. A titration is carried out for 25.00 ml of 0.100. Titration Curve Naoh.
From www.researchgate.net
A) Titration curve of the RE 2 m ZnSO4 titrating it with 0.1 m NaOH and Titration Curve Naoh What are ph titration curves. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). The volume of the titrant added. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown. Titration Curve Naoh.
From www.chegg.com
Solved The Curve Shows The Titration Of H2SO3 With NaOH. Titration Curve Naoh A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). The steep portion of the curve prior to the equivalence point is short. The volume of the titrant added. Titration is a technique used in neutralisation reactions between acids and. Titration Curve Naoh.
From www.transtutors.com
(Solved) Shown below is the titration curve of glycine (Gly), using Titration Curve Naoh What are ph titration curves. The titration of a weak acid with strong base. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). It usually only occurs until a ph of around 10. The image of a titration curve. Titration Curve Naoh.
From plot.ly
Titration Curve of Maleic Acid with NaOH Solution scatter chart made Titration Curve Naoh A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; All of the characteristics described above can be seen within. Titration Curve Naoh.
From pubs.sciepub.com
Figure 5B. Plot of the titration of strong acid (HCl= 0.1M) with strong Titration Curve Naoh The titration of a weak acid with strong base. The image of a titration curve of a weak acid with a strong base is seen below. All of the characteristics described above can be seen within it. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the.. Titration Curve Naoh.
From www.chemistryscl.com
NaOH and HCl Titration Curves Selecting Indicators Titration Curve Naoh A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). The image of a titration curve of a weak acid with a strong base is seen below. All of the characteristics described above can be seen within it. Titration is. Titration Curve Naoh.
From www.chegg.com
Titration Curve H2SO4/NaOH At each point in the Titration Curve Naoh A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). The steep portion of the curve prior to the equivalence point is short. The volume of the titrant added. The titration of a weak acid with strong base. What are. Titration Curve Naoh.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curve Naoh The titration of a weak acid with strong base. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). The image of a titration curve of a weak acid with a strong base is seen below. What are ph titration. Titration Curve Naoh.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base Titration Curve Naoh Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; It usually only occurs until a ph of around 10. The image of a titration curve of a weak acid with a strong base is seen below. The volume of the titrant added. The steep portion of the curve prior. Titration Curve Naoh.
From mungfali.com
Titration Curve HCl And NaOH Titration Curve Naoh It usually only occurs until a ph of around 10. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; All of the characteristics described above can be seen within it. The volume of the titrant added. A titration is carried out for 25.00 ml of 0.100 m hcl (strong. Titration Curve Naoh.
From chart-studio.plotly.com
Titration Curve(pH vs Volume of NaOH) line chart made by 7rat2 plotly Titration Curve Naoh The steep portion of the curve prior to the equivalence point is short. What are ph titration curves. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. The titration of a weak acid with strong base. All of the characteristics described above can be seen within. Titration Curve Naoh.
From psu.pb.unizin.org
AcidBase Titrations (14.7) Chemistry 110 Titration Curve Naoh It usually only occurs until a ph of around 10. The steep portion of the curve prior to the equivalence point is short. The image of a titration curve of a weak acid with a strong base is seen below. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution;. Titration Curve Naoh.
From socratic.org
What is the Ka value of this citric acid + NaOH titration? Socratic Titration Curve Naoh The image of a titration curve of a weak acid with a strong base is seen below. What are ph titration curves. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). The steep portion of the curve prior to. Titration Curve Naoh.
From www.youtube.com
Conductometric titration I strong acid (HCl) versus strong base Titration Curve Naoh The titration of a weak acid with strong base. The image of a titration curve of a weak acid with a strong base is seen below. All of the characteristics described above can be seen within it. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; The volume of. Titration Curve Naoh.
From www.chegg.com
Solved HCl NaOH Titration Curve CH3COOH NaOH Titration Titration Curve Naoh It usually only occurs until a ph of around 10. All of the characteristics described above can be seen within it. The titration of a weak acid with strong base. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18).. Titration Curve Naoh.
From www.chegg.com
Titration of sulfuric acid Average Volume of NaOH = Titration Curve Naoh Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. The volume of the titrant added. The image of a titration curve of a weak acid with a strong base is seen below. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid). Titration Curve Naoh.