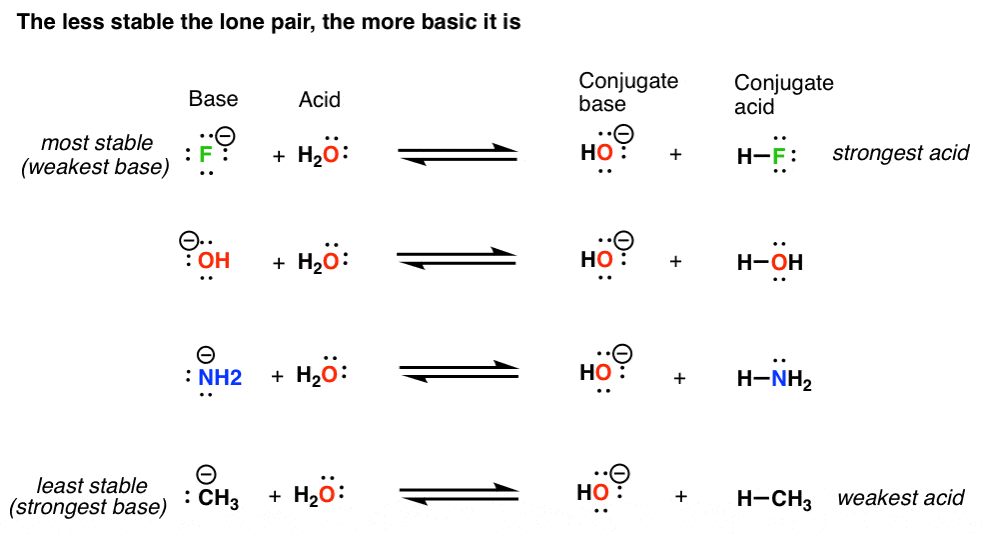
How To Tell If A Conjugate Base Is Strong . In the conjugate base of ethane, the negative charge is borne by a carbon atom, while. What is the conjugate acid or the conjugate base of (a) hcl; Look at where the negative charge ends up in each conjugate base. (b) ch 3 nh 2; Strong bases have a weak conjugate acid. How would you identify the acid, base, conjugate acid and conjugate base in the following reaction: Hcl is a strong acid. Let's consider the relationship between the strength of the ammonium (nh 4 +) and its conjugate base, ammonia (nh 3). The first six acids in figure 13.1.3.2. In this chart, the strongest acids are at the top left, and the weakest bases are at the top right. Strong acids form very weak conjugate bases, and weak acids form stronger conjugate bases (figure 13.1.3.2).
from www.masterorganicchemistry.com
What is the conjugate acid or the conjugate base of (a) hcl; Look at where the negative charge ends up in each conjugate base. In this chart, the strongest acids are at the top left, and the weakest bases are at the top right. (b) ch 3 nh 2; In the conjugate base of ethane, the negative charge is borne by a carbon atom, while. How would you identify the acid, base, conjugate acid and conjugate base in the following reaction: Let's consider the relationship between the strength of the ammonium (nh 4 +) and its conjugate base, ammonia (nh 3). Strong acids form very weak conjugate bases, and weak acids form stronger conjugate bases (figure 13.1.3.2). The first six acids in figure 13.1.3.2. Strong bases have a weak conjugate acid.
Basicity Is Another Word For "Stability Of A Lone Pair Of Electrons"
How To Tell If A Conjugate Base Is Strong Strong bases have a weak conjugate acid. In the conjugate base of ethane, the negative charge is borne by a carbon atom, while. Hcl is a strong acid. Look at where the negative charge ends up in each conjugate base. The first six acids in figure 13.1.3.2. Strong bases have a weak conjugate acid. In this chart, the strongest acids are at the top left, and the weakest bases are at the top right. What is the conjugate acid or the conjugate base of (a) hcl; Let's consider the relationship between the strength of the ammonium (nh 4 +) and its conjugate base, ammonia (nh 3). Strong acids form very weak conjugate bases, and weak acids form stronger conjugate bases (figure 13.1.3.2). How would you identify the acid, base, conjugate acid and conjugate base in the following reaction: (b) ch 3 nh 2;
From www.facebook.com
Worship Service 11/3/2024 How To Tell If A Conjugate Base Is Strong In the conjugate base of ethane, the negative charge is borne by a carbon atom, while. (b) ch 3 nh 2; Let's consider the relationship between the strength of the ammonium (nh 4 +) and its conjugate base, ammonia (nh 3). In this chart, the strongest acids are at the top left, and the weakest bases are at the top. How To Tell If A Conjugate Base Is Strong.
From smithinart1960.blogspot.com
How to Effectively Read a Pka Table Smith Inart1960 How To Tell If A Conjugate Base Is Strong Let's consider the relationship between the strength of the ammonium (nh 4 +) and its conjugate base, ammonia (nh 3). Look at where the negative charge ends up in each conjugate base. What is the conjugate acid or the conjugate base of (a) hcl; Hcl is a strong acid. In the conjugate base of ethane, the negative charge is borne. How To Tell If A Conjugate Base Is Strong.
From proper-cooking.info
Ph Examples Of Acids And Bases How To Tell If A Conjugate Base Is Strong In the conjugate base of ethane, the negative charge is borne by a carbon atom, while. Let's consider the relationship between the strength of the ammonium (nh 4 +) and its conjugate base, ammonia (nh 3). Hcl is a strong acid. What is the conjugate acid or the conjugate base of (a) hcl; (b) ch 3 nh 2; How would. How To Tell If A Conjugate Base Is Strong.
From www.facebook.com
Worship Service 11/3/2024 How To Tell If A Conjugate Base Is Strong Strong bases have a weak conjugate acid. What is the conjugate acid or the conjugate base of (a) hcl; Look at where the negative charge ends up in each conjugate base. Strong acids form very weak conjugate bases, and weak acids form stronger conjugate bases (figure 13.1.3.2). Let's consider the relationship between the strength of the ammonium (nh 4 +). How To Tell If A Conjugate Base Is Strong.
From www.youtube.com
How to determine Strong Acids Conjugate Base, pKa, Electronegative How To Tell If A Conjugate Base Is Strong Strong acids form very weak conjugate bases, and weak acids form stronger conjugate bases (figure 13.1.3.2). Let's consider the relationship between the strength of the ammonium (nh 4 +) and its conjugate base, ammonia (nh 3). Hcl is a strong acid. How would you identify the acid, base, conjugate acid and conjugate base in the following reaction: Strong bases have. How To Tell If A Conjugate Base Is Strong.
From ar.inspiredpencil.com
Strong Acid And Conjugate Base How To Tell If A Conjugate Base Is Strong In the conjugate base of ethane, the negative charge is borne by a carbon atom, while. Let's consider the relationship between the strength of the ammonium (nh 4 +) and its conjugate base, ammonia (nh 3). Hcl is a strong acid. The first six acids in figure 13.1.3.2. Strong acids form very weak conjugate bases, and weak acids form stronger. How To Tell If A Conjugate Base Is Strong.
From www.masterorganicchemistry.com
What Makes A Good Nucleophile? Master Organic Chemistry How To Tell If A Conjugate Base Is Strong How would you identify the acid, base, conjugate acid and conjugate base in the following reaction: Look at where the negative charge ends up in each conjugate base. What is the conjugate acid or the conjugate base of (a) hcl; In this chart, the strongest acids are at the top left, and the weakest bases are at the top right.. How To Tell If A Conjugate Base Is Strong.
From ar.inspiredpencil.com
Strong Acid And Conjugate Base How To Tell If A Conjugate Base Is Strong The first six acids in figure 13.1.3.2. Strong bases have a weak conjugate acid. (b) ch 3 nh 2; Hcl is a strong acid. Look at where the negative charge ends up in each conjugate base. Strong acids form very weak conjugate bases, and weak acids form stronger conjugate bases (figure 13.1.3.2). In the conjugate base of ethane, the negative. How To Tell If A Conjugate Base Is Strong.
From www.expii.com
Conjugate AcidBase Pairs — Overview & Examples Expii How To Tell If A Conjugate Base Is Strong How would you identify the acid, base, conjugate acid and conjugate base in the following reaction: Look at where the negative charge ends up in each conjugate base. Hcl is a strong acid. What is the conjugate acid or the conjugate base of (a) hcl; In the conjugate base of ethane, the negative charge is borne by a carbon atom,. How To Tell If A Conjugate Base Is Strong.
From www.coursehero.com
[Solved] Calculate the ratio of [conjugate base]/[acid] present at a pH How To Tell If A Conjugate Base Is Strong The first six acids in figure 13.1.3.2. How would you identify the acid, base, conjugate acid and conjugate base in the following reaction: Hcl is a strong acid. What is the conjugate acid or the conjugate base of (a) hcl; Strong acids form very weak conjugate bases, and weak acids form stronger conjugate bases (figure 13.1.3.2). In this chart, the. How To Tell If A Conjugate Base Is Strong.
From general.chemistrysteps.com
Conjugate Acid and Conjugate Base Chemistry Steps How To Tell If A Conjugate Base Is Strong Strong acids form very weak conjugate bases, and weak acids form stronger conjugate bases (figure 13.1.3.2). Let's consider the relationship between the strength of the ammonium (nh 4 +) and its conjugate base, ammonia (nh 3). The first six acids in figure 13.1.3.2. How would you identify the acid, base, conjugate acid and conjugate base in the following reaction: Hcl. How To Tell If A Conjugate Base Is Strong.
From larryeclloyd.blogspot.com
Conjugate Acid Base Pair LarryecLloyd How To Tell If A Conjugate Base Is Strong What is the conjugate acid or the conjugate base of (a) hcl; How would you identify the acid, base, conjugate acid and conjugate base in the following reaction: Hcl is a strong acid. (b) ch 3 nh 2; Look at where the negative charge ends up in each conjugate base. In this chart, the strongest acids are at the top. How To Tell If A Conjugate Base Is Strong.
From study.com
How to Determine Conjugate Acid or Base Strength Chemistry How To Tell If A Conjugate Base Is Strong How would you identify the acid, base, conjugate acid and conjugate base in the following reaction: In this chart, the strongest acids are at the top left, and the weakest bases are at the top right. The first six acids in figure 13.1.3.2. What is the conjugate acid or the conjugate base of (a) hcl; Let's consider the relationship between. How To Tell If A Conjugate Base Is Strong.
From ecampusontario.pressbooks.pub
5.1 AcidBase Definitions & Conjugate AcidBase Pairs General How To Tell If A Conjugate Base Is Strong In the conjugate base of ethane, the negative charge is borne by a carbon atom, while. The first six acids in figure 13.1.3.2. Let's consider the relationship between the strength of the ammonium (nh 4 +) and its conjugate base, ammonia (nh 3). What is the conjugate acid or the conjugate base of (a) hcl; In this chart, the strongest. How To Tell If A Conjugate Base Is Strong.
From worksheetmagiccriswell.z13.web.core.windows.net
List Of Conjugate Acids And Bases How To Tell If A Conjugate Base Is Strong The first six acids in figure 13.1.3.2. In the conjugate base of ethane, the negative charge is borne by a carbon atom, while. What is the conjugate acid or the conjugate base of (a) hcl; (b) ch 3 nh 2; Strong acids form very weak conjugate bases, and weak acids form stronger conjugate bases (figure 13.1.3.2). In this chart, the. How To Tell If A Conjugate Base Is Strong.
From ar.inspiredpencil.com
Examples Of Bases How To Tell If A Conjugate Base Is Strong How would you identify the acid, base, conjugate acid and conjugate base in the following reaction: Let's consider the relationship between the strength of the ammonium (nh 4 +) and its conjugate base, ammonia (nh 3). In this chart, the strongest acids are at the top left, and the weakest bases are at the top right. What is the conjugate. How To Tell If A Conjugate Base Is Strong.
From www.chemicals.co.uk
What Is Conjugation In Chemistry? The Chemistry Blog How To Tell If A Conjugate Base Is Strong Hcl is a strong acid. In the conjugate base of ethane, the negative charge is borne by a carbon atom, while. In this chart, the strongest acids are at the top left, and the weakest bases are at the top right. (b) ch 3 nh 2; Let's consider the relationship between the strength of the ammonium (nh 4 +) and. How To Tell If A Conjugate Base Is Strong.
From www.masterorganicchemistry.com
Basicity Is Another Word For "Stability Of A Lone Pair Of Electrons" How To Tell If A Conjugate Base Is Strong Hcl is a strong acid. Strong acids form very weak conjugate bases, and weak acids form stronger conjugate bases (figure 13.1.3.2). Look at where the negative charge ends up in each conjugate base. In this chart, the strongest acids are at the top left, and the weakest bases are at the top right. What is the conjugate acid or the. How To Tell If A Conjugate Base Is Strong.
From www.organicchemistrytutor.com
BronstedLowry Theory — Organic Chemistry Tutor How To Tell If A Conjugate Base Is Strong In this chart, the strongest acids are at the top left, and the weakest bases are at the top right. Hcl is a strong acid. What is the conjugate acid or the conjugate base of (a) hcl; Strong acids form very weak conjugate bases, and weak acids form stronger conjugate bases (figure 13.1.3.2). The first six acids in figure 13.1.3.2.. How To Tell If A Conjugate Base Is Strong.
From www.facebook.com
Worship Service 11/3/2024 How To Tell If A Conjugate Base Is Strong Strong bases have a weak conjugate acid. Hcl is a strong acid. What is the conjugate acid or the conjugate base of (a) hcl; Let's consider the relationship between the strength of the ammonium (nh 4 +) and its conjugate base, ammonia (nh 3). In this chart, the strongest acids are at the top left, and the weakest bases are. How To Tell If A Conjugate Base Is Strong.
From englishlinx.com
Verbs Worksheets Verb Conjugation Worksheets How To Tell If A Conjugate Base Is Strong (b) ch 3 nh 2; In this chart, the strongest acids are at the top left, and the weakest bases are at the top right. Strong bases have a weak conjugate acid. How would you identify the acid, base, conjugate acid and conjugate base in the following reaction: What is the conjugate acid or the conjugate base of (a) hcl;. How To Tell If A Conjugate Base Is Strong.
From www.youtube.com
How to identify conjugate acidbase pairs. YouTube How To Tell If A Conjugate Base Is Strong Hcl is a strong acid. How would you identify the acid, base, conjugate acid and conjugate base in the following reaction: Strong bases have a weak conjugate acid. The first six acids in figure 13.1.3.2. Look at where the negative charge ends up in each conjugate base. Strong acids form very weak conjugate bases, and weak acids form stronger conjugate. How To Tell If A Conjugate Base Is Strong.
From slideplayer.com
Strength of Acids and Bases ppt download How To Tell If A Conjugate Base Is Strong Look at where the negative charge ends up in each conjugate base. Hcl is a strong acid. (b) ch 3 nh 2; The first six acids in figure 13.1.3.2. Strong bases have a weak conjugate acid. In this chart, the strongest acids are at the top left, and the weakest bases are at the top right. How would you identify. How To Tell If A Conjugate Base Is Strong.
From dxoeyowlb.blob.core.windows.net
Equivalence Point Base Titration at Myrna Sanchez blog How To Tell If A Conjugate Base Is Strong The first six acids in figure 13.1.3.2. Look at where the negative charge ends up in each conjugate base. (b) ch 3 nh 2; In the conjugate base of ethane, the negative charge is borne by a carbon atom, while. How would you identify the acid, base, conjugate acid and conjugate base in the following reaction: Strong acids form very. How To Tell If A Conjugate Base Is Strong.
From ar.inspiredpencil.com
H2so4 Lewis Structure Conjugate Base How To Tell If A Conjugate Base Is Strong In the conjugate base of ethane, the negative charge is borne by a carbon atom, while. What is the conjugate acid or the conjugate base of (a) hcl; (b) ch 3 nh 2; Hcl is a strong acid. Strong acids form very weak conjugate bases, and weak acids form stronger conjugate bases (figure 13.1.3.2). Look at where the negative charge. How To Tell If A Conjugate Base Is Strong.
From www.masterorganicchemistry.com
How To Use a pKa Table How To Tell If A Conjugate Base Is Strong Hcl is a strong acid. (b) ch 3 nh 2; Let's consider the relationship between the strength of the ammonium (nh 4 +) and its conjugate base, ammonia (nh 3). How would you identify the acid, base, conjugate acid and conjugate base in the following reaction: Strong acids form very weak conjugate bases, and weak acids form stronger conjugate bases. How To Tell If A Conjugate Base Is Strong.
From www.myxxgirl.com
Figure Ab The Pka Scale And Its Effect On Conjugate Bases My XXX Hot Girl How To Tell If A Conjugate Base Is Strong What is the conjugate acid or the conjugate base of (a) hcl; Strong bases have a weak conjugate acid. The first six acids in figure 13.1.3.2. (b) ch 3 nh 2; How would you identify the acid, base, conjugate acid and conjugate base in the following reaction: Hcl is a strong acid. In the conjugate base of ethane, the negative. How To Tell If A Conjugate Base Is Strong.
From ar.inspiredpencil.com
Strong Acid And Conjugate Base How To Tell If A Conjugate Base Is Strong Hcl is a strong acid. Let's consider the relationship between the strength of the ammonium (nh 4 +) and its conjugate base, ammonia (nh 3). In the conjugate base of ethane, the negative charge is borne by a carbon atom, while. How would you identify the acid, base, conjugate acid and conjugate base in the following reaction: The first six. How To Tell If A Conjugate Base Is Strong.
From wiki.americamakes.us
Conjugate Acids And Bases Worksheet How To Tell If A Conjugate Base Is Strong The first six acids in figure 13.1.3.2. Strong acids form very weak conjugate bases, and weak acids form stronger conjugate bases (figure 13.1.3.2). (b) ch 3 nh 2; In the conjugate base of ethane, the negative charge is borne by a carbon atom, while. Hcl is a strong acid. How would you identify the acid, base, conjugate acid and conjugate. How To Tell If A Conjugate Base Is Strong.
From quizzschooltalliates.z21.web.core.windows.net
How To Identify Acids And Bases How To Tell If A Conjugate Base Is Strong How would you identify the acid, base, conjugate acid and conjugate base in the following reaction: Hcl is a strong acid. In the conjugate base of ethane, the negative charge is borne by a carbon atom, while. Let's consider the relationship between the strength of the ammonium (nh 4 +) and its conjugate base, ammonia (nh 3). Strong acids form. How To Tell If A Conjugate Base Is Strong.
From www.expii.com
Conjugate AcidBase Pairs — Overview & Examples Expii How To Tell If A Conjugate Base Is Strong The first six acids in figure 13.1.3.2. (b) ch 3 nh 2; How would you identify the acid, base, conjugate acid and conjugate base in the following reaction: Let's consider the relationship between the strength of the ammonium (nh 4 +) and its conjugate base, ammonia (nh 3). What is the conjugate acid or the conjugate base of (a) hcl;. How To Tell If A Conjugate Base Is Strong.
From courses.lumenlearning.com
Relative Strengths of Acids and Bases Chemistry How To Tell If A Conjugate Base Is Strong How would you identify the acid, base, conjugate acid and conjugate base in the following reaction: (b) ch 3 nh 2; In this chart, the strongest acids are at the top left, and the weakest bases are at the top right. In the conjugate base of ethane, the negative charge is borne by a carbon atom, while. Strong acids form. How To Tell If A Conjugate Base Is Strong.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download How To Tell If A Conjugate Base Is Strong (b) ch 3 nh 2; Let's consider the relationship between the strength of the ammonium (nh 4 +) and its conjugate base, ammonia (nh 3). How would you identify the acid, base, conjugate acid and conjugate base in the following reaction: Strong acids form very weak conjugate bases, and weak acids form stronger conjugate bases (figure 13.1.3.2). In the conjugate. How To Tell If A Conjugate Base Is Strong.
From www.myxxgirl.com
Ranking Acids And Bases Using Ka And Pka Values My XXX Hot Girl How To Tell If A Conjugate Base Is Strong What is the conjugate acid or the conjugate base of (a) hcl; Hcl is a strong acid. In the conjugate base of ethane, the negative charge is borne by a carbon atom, while. Strong acids form very weak conjugate bases, and weak acids form stronger conjugate bases (figure 13.1.3.2). Look at where the negative charge ends up in each conjugate. How To Tell If A Conjugate Base Is Strong.
From www.youtube.com
8.4 Strong Acids have Weak Conjugate Bases [SL Chemistry] YouTube How To Tell If A Conjugate Base Is Strong How would you identify the acid, base, conjugate acid and conjugate base in the following reaction: Strong acids form very weak conjugate bases, and weak acids form stronger conjugate bases (figure 13.1.3.2). Look at where the negative charge ends up in each conjugate base. (b) ch 3 nh 2; The first six acids in figure 13.1.3.2. Hcl is a strong. How To Tell If A Conjugate Base Is Strong.