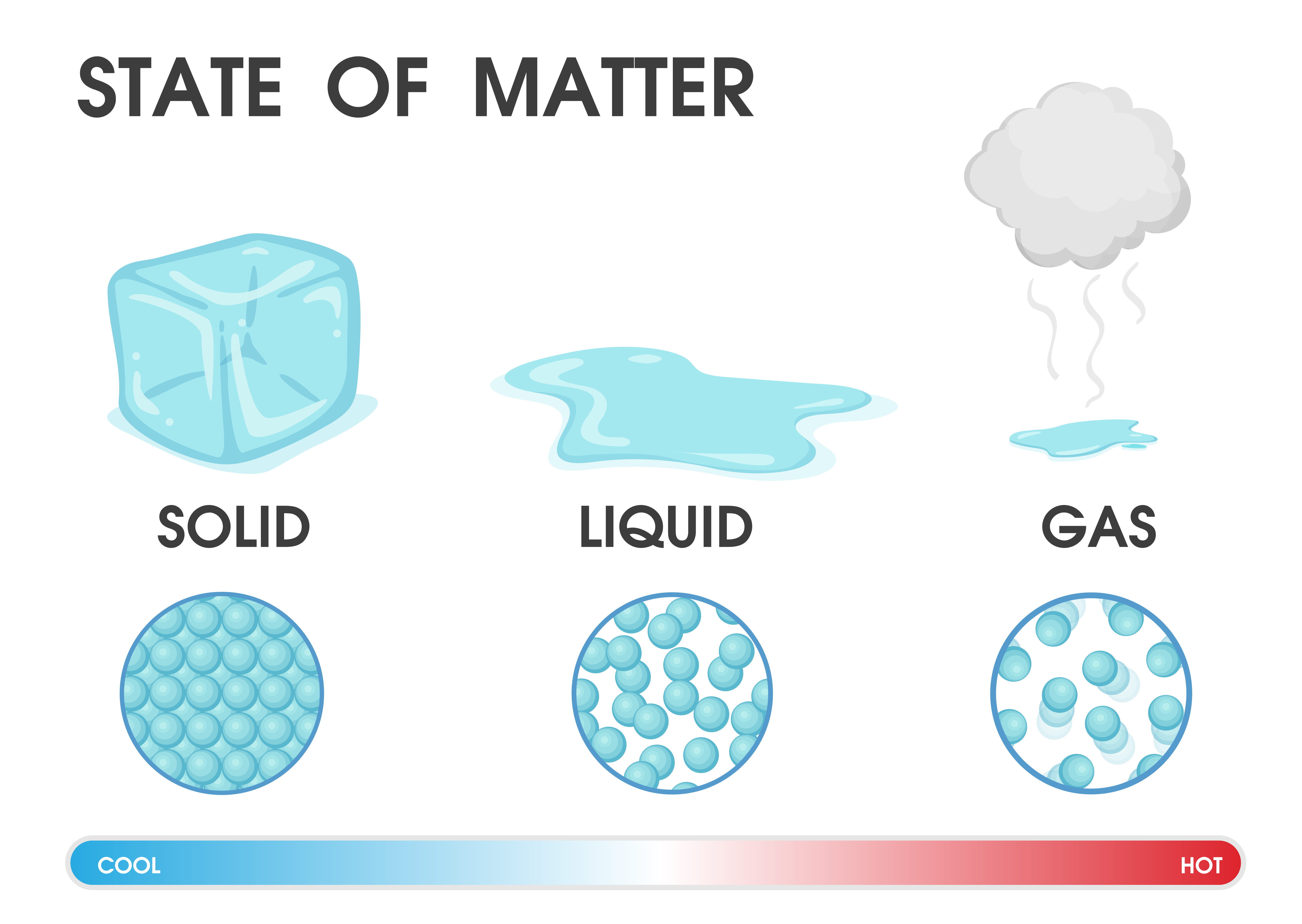
Is Solid To Liquid Boiling . Sublimation is isothermal, like the other phase changes. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly become a solid. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). A phase of a thermodynamic system and the states of. Use of one term or the other is normally dictated by the direction of the phase transition being considered, for example, solid to liquid. There are four main changes of state: Commonly the term is used to refer to changes among the basic states of matter: Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. The conversion of a solid to a liquid is called fusion (or melting). Solid, liquid, and gas, and in rare cases, plasma.
from manualparttrieste88.z22.web.core.windows.net
Sublimation is isothermal, like the other phase changes. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly become a solid. Solid, liquid, and gas, and in rare cases, plasma. Commonly the term is used to refer to changes among the basic states of matter: There are four main changes of state: Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. Use of one term or the other is normally dictated by the direction of the phase transition being considered, for example, solid to liquid. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The conversion of a solid to a liquid is called fusion (or melting). A phase of a thermodynamic system and the states of.
Solid Liquid Gas Diagram
Is Solid To Liquid Boiling Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. Sublimation is isothermal, like the other phase changes. There are four main changes of state: A phase of a thermodynamic system and the states of. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly become a solid. Use of one term or the other is normally dictated by the direction of the phase transition being considered, for example, solid to liquid. Solid, liquid, and gas, and in rare cases, plasma. The conversion of a solid to a liquid is called fusion (or melting). Commonly the term is used to refer to changes among the basic states of matter:
From exymotkoo.blob.core.windows.net
Solid And Liquid Phases In Equilibrium at Jessica Florian blog Is Solid To Liquid Boiling The conversion of a solid to a liquid is called fusion (or melting). The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Solid, liquid, and gas, and in rare cases, plasma. Sublimation is isothermal, like the other phase changes. Under some circumstances, the solid phase can transition directly to the gas phase. Is Solid To Liquid Boiling.
From www.bajeczneobrazy.pl
Physical states of matter.Solid, liquid and gas.Melting, freezing Is Solid To Liquid Boiling The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). There are four main changes of state: A phase of a thermodynamic system and the states of. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. Solid, liquid, and gas, and in. Is Solid To Liquid Boiling.
From primaryleap.co.uk
Chemistry Separating Mixtures Level 1 activity for kids PrimaryLeap Is Solid To Liquid Boiling Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly become a solid. There are four main changes of state: Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. A phase of a thermodynamic system. Is Solid To Liquid Boiling.
From giobjqxrq.blob.core.windows.net
Solid Liquid Gas Transition at Pauline Lang blog Is Solid To Liquid Boiling The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Solid, liquid, and gas, and in rare cases, plasma. Use of one term or the other is normally dictated by the direction of the phase transition being considered, for example, solid to liquid. Sublimation is isothermal, like the other phase changes. A phase. Is Solid To Liquid Boiling.
From igcsechemistryrevision.weebly.com
iGCSE CHEMISTRY REVISION HELP Particles & Equations Is Solid To Liquid Boiling Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly become a solid. Commonly the term is used to refer to changes among the basic states of matter: Use of one term or the other is normally dictated by the direction of the phase transition being. Is Solid To Liquid Boiling.
From loeaanczy.blob.core.windows.net
Chlorine Boiling Point And Melting Point at Jane Gibbs blog Is Solid To Liquid Boiling The conversion of a solid to a liquid is called fusion (or melting). Sublimation is isothermal, like the other phase changes. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly become a solid. A phase of a thermodynamic system and the states of. There are. Is Solid To Liquid Boiling.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock Is Solid To Liquid Boiling The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). A phase of a thermodynamic system and the states of. The conversion of a solid to a liquid is called fusion (or melting). Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. Is Solid To Liquid Boiling.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox Is Solid To Liquid Boiling The conversion of a solid to a liquid is called fusion (or melting). Sublimation is isothermal, like the other phase changes. A phase of a thermodynamic system and the states of. Use of one term or the other is normally dictated by the direction of the phase transition being considered, for example, solid to liquid. There are four main changes. Is Solid To Liquid Boiling.
From slideplayer.com
Phases of Matter. ppt download Is Solid To Liquid Boiling There are four main changes of state: Use of one term or the other is normally dictated by the direction of the phase transition being considered, for example, solid to liquid. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly become a solid. Sublimation is. Is Solid To Liquid Boiling.
From studylib.net
Boiling Point = Temperature at which a liquid turns into a gas Is Solid To Liquid Boiling There are four main changes of state: Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly become a solid. The conversion of a solid to a liquid is called fusion (or melting). The energy required to melt 1 mol of a substance is its enthalpy. Is Solid To Liquid Boiling.
From general.chemistrysteps.com
Entropy and State Change Chemistry Steps Is Solid To Liquid Boiling Commonly the term is used to refer to changes among the basic states of matter: Sublimation is isothermal, like the other phase changes. The conversion of a solid to a liquid is called fusion (or melting). Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly. Is Solid To Liquid Boiling.
From www.slideserve.com
PPT Boiling Point Notes PowerPoint Presentation, free download ID Is Solid To Liquid Boiling There are four main changes of state: Solid, liquid, and gas, and in rare cases, plasma. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Sublimation is isothermal, like the other phase changes. Commonly the term is used to refer to changes among the basic states of matter: A phase of a. Is Solid To Liquid Boiling.
From www.peoi.org
Chapter 10 Section C Properties of Liquids Is Solid To Liquid Boiling The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Use of one term or the other is normally dictated by the direction of the phase transition being considered, for example, solid to liquid. A phase of a thermodynamic system and the states of. Solid, liquid, and gas, and in rare cases, plasma.. Is Solid To Liquid Boiling.
From slideplayer.com
Classifying Matter. ppt download Is Solid To Liquid Boiling Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. Solid, liquid, and gas, and in rare cases, plasma. Sublimation is isothermal, like the other phase changes. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Use of one term or the. Is Solid To Liquid Boiling.
From gabriela-classwork.blogspot.com
Science Class septiembre 2010 Is Solid To Liquid Boiling A phase of a thermodynamic system and the states of. Commonly the term is used to refer to changes among the basic states of matter: Sublimation is isothermal, like the other phase changes. There are four main changes of state: Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. Is Solid To Liquid Boiling.
From slideplayer.com
Phases of Matter (chapter 13+14) ppt download Is Solid To Liquid Boiling A phase of a thermodynamic system and the states of. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly become a solid. Use of one. Is Solid To Liquid Boiling.
From favpng.com
Boiling Point Melting Point Heat Temperature Chemistry, PNG Is Solid To Liquid Boiling A phase of a thermodynamic system and the states of. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly become a solid. There are four main changes of state: Sublimation is isothermal, like the other phase changes. Use of one term or the other is. Is Solid To Liquid Boiling.
From socratic.org
Question 15820 Socratic Is Solid To Liquid Boiling A phase of a thermodynamic system and the states of. Use of one term or the other is normally dictated by the direction of the phase transition being considered, for example, solid to liquid. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Commonly the term is used to refer to changes. Is Solid To Liquid Boiling.
From exycvcnnl.blob.core.windows.net
Solids Liquids And Gases Lesson 1 at Vanessa Crum blog Is Solid To Liquid Boiling The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly become a solid. Use of one term or the other is normally dictated by the direction of the phase. Is Solid To Liquid Boiling.
From johnwest.edublogs.org
Solids liquids and gasses, a story in three parts. Science Connected Is Solid To Liquid Boiling Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly become a solid. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. A phase of a thermodynamic system and the states of. Use of one. Is Solid To Liquid Boiling.
From vhmsscience.weebly.com
Phase Change Boiling Water Lab VISTA HEIGHTS 8TH GRADE SCIENCE Is Solid To Liquid Boiling Solid, liquid, and gas, and in rare cases, plasma. Commonly the term is used to refer to changes among the basic states of matter: Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. Use of one term or the other is normally dictated by the direction of the phase. Is Solid To Liquid Boiling.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox Is Solid To Liquid Boiling There are four main changes of state: The conversion of a solid to a liquid is called fusion (or melting). Commonly the term is used to refer to changes among the basic states of matter: Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. The energy required to melt. Is Solid To Liquid Boiling.
From owlcation.com
What Are the Freezing, Melting, and Boiling Points of Solids, Liquids Is Solid To Liquid Boiling Solid, liquid, and gas, and in rare cases, plasma. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Commonly the term is used to refer to changes among the basic states of matter: Use of one term or the other is normally dictated by the direction of the phase transition being considered,. Is Solid To Liquid Boiling.
From manualparttrieste88.z22.web.core.windows.net
Solid Liquid Gas Diagram Is Solid To Liquid Boiling The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). A phase of a thermodynamic system and the states of. Use of one term or the other is normally dictated by the direction of the phase transition being considered, for example, solid to liquid. Under some circumstances, the solid phase can transition directly. Is Solid To Liquid Boiling.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk Is Solid To Liquid Boiling Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. Sublimation is isothermal, like the other phase changes. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). There are four main changes of state: A phase of a thermodynamic system and the. Is Solid To Liquid Boiling.
From fyomtaojv.blob.core.windows.net
Solid Liquid Gas Changes Of State at Martha Neece blog Is Solid To Liquid Boiling Sublimation is isothermal, like the other phase changes. The conversion of a solid to a liquid is called fusion (or melting). Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly become a solid. Under some circumstances, the solid phase can transition directly to the gas. Is Solid To Liquid Boiling.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Is Solid To Liquid Boiling The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Use of one term or the other is normally dictated by the direction of the phase transition being considered, for example, solid to liquid. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase,. Is Solid To Liquid Boiling.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Is Solid To Liquid Boiling Solid, liquid, and gas, and in rare cases, plasma. There are four main changes of state: Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly become a solid. Under some circumstances, the solid phase can transition directly to the gas phase without going through a. Is Solid To Liquid Boiling.
From giokinxez.blob.core.windows.net
Copper Element Solid Liquid Or Gas at Archie Adamski blog Is Solid To Liquid Boiling There are four main changes of state: Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly become a solid. Sublimation is isothermal, like the other. Is Solid To Liquid Boiling.
From exylluyva.blob.core.windows.net
Solid Liquid Gas Melting Point Boiling Point at Margaret Chaffins blog Is Solid To Liquid Boiling Solid, liquid, and gas, and in rare cases, plasma. Commonly the term is used to refer to changes among the basic states of matter: The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase,. Is Solid To Liquid Boiling.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Is Solid To Liquid Boiling Solid, liquid, and gas, and in rare cases, plasma. The conversion of a solid to a liquid is called fusion (or melting). Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid. Is Solid To Liquid Boiling.
From www.sciencelearn.org.nz
Solid to liquid to gas — Science Learning Hub Is Solid To Liquid Boiling Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Solid, liquid, and gas, and in rare cases, plasma. A phase of a thermodynamic system and the states of. Use of one term. Is Solid To Liquid Boiling.
From exykjlgyk.blob.core.windows.net
Three Examples Each Of Solids Liquids And Gases at Meghan Anderson blog Is Solid To Liquid Boiling The conversion of a solid to a liquid is called fusion (or melting). Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. Use of one term or the other is normally dictated by the direction of the phase transition being considered, for example, solid to liquid. Sublimation is isothermal,. Is Solid To Liquid Boiling.
From www.researchgate.net
Preparation of the flow chart of SiS 2 . (a) Solid−liquid boiling Is Solid To Liquid Boiling Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly become a solid. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). There are four main changes of state: Sublimation is isothermal, like the other phase changes. Use. Is Solid To Liquid Boiling.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs Is Solid To Liquid Boiling The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). A phase of a thermodynamic system and the states of. There are four main changes of state: The conversion of a solid to a liquid is called fusion (or melting). Solid, liquid, and gas, and in rare cases, plasma. Sublimation is isothermal, like. Is Solid To Liquid Boiling.