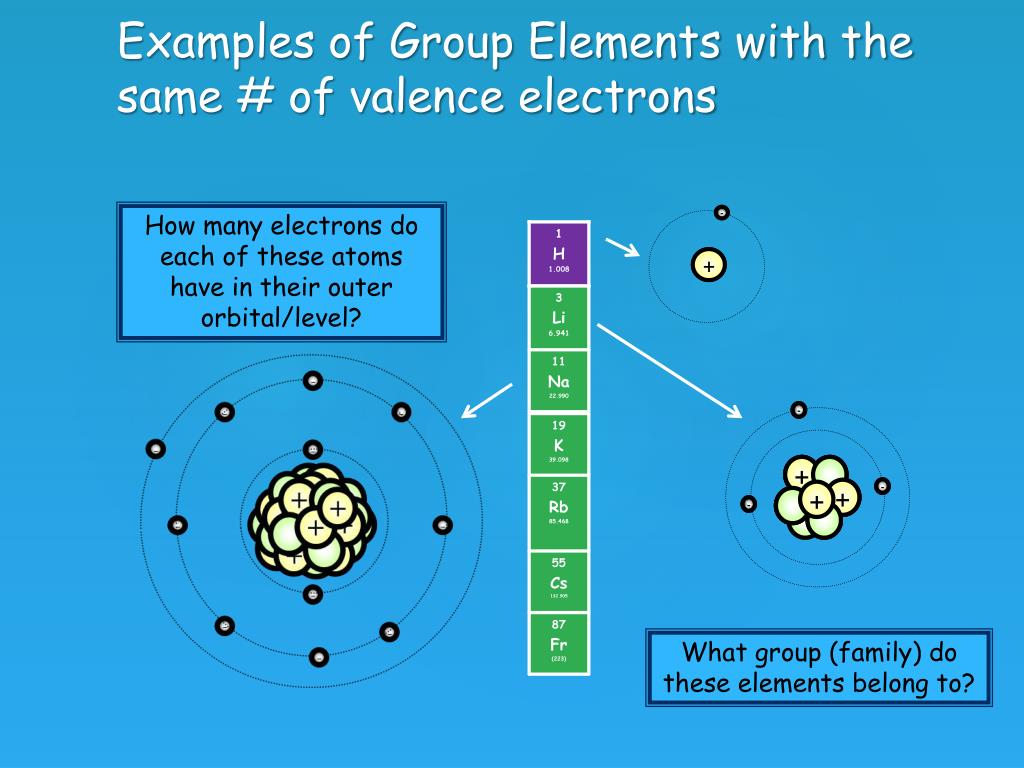
Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With . chlorine has 7 electrons in its valence shell. this means that calcium #s^2# has 2 valence electrons it readily gives away in order to seek the stability of the octet. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is the central atom. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven. To meet the octet rule, it must either gain one electron or lose seven. chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic table, also.
from www.slideserve.com
the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven. this means that calcium #s^2# has 2 valence electrons it readily gives away in order to seek the stability of the octet. chlorine has 7 electrons in its valence shell. To meet the octet rule, it must either gain one electron or lose seven. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is the central atom. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is. chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic table, also. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom.
PPT Valence Electrons PowerPoint Presentation, free download ID5581383
Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is. chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic table, also. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is the central atom. To meet the octet rule, it must either gain one electron or lose seven. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven. chlorine has 7 electrons in its valence shell. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is. this means that calcium #s^2# has 2 valence electrons it readily gives away in order to seek the stability of the octet. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom.
From slideplayer.com
BellRinger What are valence electrons? ppt download Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With this means that calcium #s^2# has 2 valence electrons it readily gives away in order to seek the stability of the octet. chlorine has 7 electrons in its valence shell. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is the central atom. the 3s^2 3p^5 electrons are the outermost electrons,. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From www.chegg.com
Solved Calcium has two valence electrons and an Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is the central atom. chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic table, also. this means that calcium #s^2#. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From exojqwivc.blob.core.windows.net
Do Atoms Need Electrons at Jesse Engel blog Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is the central atom. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven. chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic table, also. nitrogen is less electronegative than chlorine, and halogen. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From www.numerade.com
SOLVED Model 4 Period 3 Elements Sodium Aluminum Chlorine Locate these Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With this means that calcium #s^2# has 2 valence electrons it readily gives away in order to seek the stability of the octet. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. nitrogen is less electronegative. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From kunduz.com
[ANSWERED] The electron dot diagram for a neutral at... Physical Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic table, also. chlorine has 7 electrons in. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From valenceelectrons.com
How to Find the Valence Electrons for PCl3? Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is. chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic table, also. chlorine has 7 electrons in its valence shell. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven. To meet the. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven. chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic table, also. chlorine has 7 electrons in its valence shell. 93 rows. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From schematicsarjairllixq7.z22.web.core.windows.net
Chlorine Electric Dot Diagram Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. To meet the octet rule, it must either gain one electron or lose seven. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is the central atom. chlorine, with 7 valence electrons, most readily. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From valenceelectrons.com
How to Find the Valence Electrons for ClF3 (Chlorine Trifluoride)? Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is. chlorine has 7 electrons in its valence shell. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven. nitrogen is. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From www.researchgate.net
hypochlorous acid Chlorine has 7 valence electrons, oxygen has 6 Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With To meet the octet rule, it must either gain one electron or lose seven. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From www.transtutors.com
(Get Answer) What is the electron configuration for Cl, chlorine, and Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With this means that calcium #s^2# has 2 valence electrons it readily gives away in order to seek the stability of the octet. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. chlorine,. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven. To meet the octet rule, it must either gain one electron or lose seven. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is. chlorine has 7 electrons in its valence shell. chlorine, with 7 valence electrons, most readily. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With this means that calcium #s^2# has 2 valence electrons it readily gives away in order to seek the stability of the octet. chlorine has 7 electrons in its valence shell. To meet the octet rule, it must either gain one electron or lose seven. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven. . Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From ar.inspiredpencil.com
Periodic Table Valence Electrons Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With To meet the octet rule, it must either gain one electron or lose seven. this means that calcium #s^2# has 2 valence electrons it readily gives away in order to seek the stability of the octet. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. chlorine has 7. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From www.expii.com
Valence Electrons — Definition & Importance Expii Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With chlorine has 7 electrons in its valence shell. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. To meet the octet rule, it must either gain one electron or lose seven. chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic table,. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From byjus.com
Find out the number of valence electrons present in chlorine. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With chlorine has 7 electrons in its valence shell. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From chemistry291.blogspot.com
How Many Valence Electrons Does chlorine Have?number of valence Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With To meet the octet rule, it must either gain one electron or lose seven. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is the central atom. this means that calcium #s^2# has 2 valence electrons it readily gives away. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From www.crowlex.com
How Many Valence Electrons Does Chlorine Have Crowlex Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is the central atom. chlorine has 7 electrons in its valence shell. chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic table, also. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal,. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From valenceelectrons.com
How to Find the Valence Electrons for COCl2 (phosgene)? Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With To meet the octet rule, it must either gain one electron or lose seven. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven. chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic table, also. chlorine has 7 electrons in its valence shell. nitrogen is less electronegative. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From byjus.com
In the electron dot structure, the valence shell electrons are Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic table, also. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. To meet the octet rule, it must either gain one electron or lose seven. nitrogen is less electronegative than chlorine, and. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From valenceelectrons.com
Protons, Neutrons, Electrons for Chlorine (Cl, Cl) Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic table, also. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is the central atom. chlorine has. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From dxoatswvu.blob.core.windows.net
Chlorine Valence Atom at Connie Downs blog Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is the central atom. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic table, also. the 3s^2. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From quizdislodging.z4.web.core.windows.net
What Element Has 7 Electrons Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is the central atom. chlorine has 7 electrons in its valence shell. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. To meet the octet rule, it must either gain one electron or lose. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With To meet the octet rule, it must either gain one electron or lose seven. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is the central atom. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven. chlorine, with 7 valence electrons, most readily bonds with elements from group 1a. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From science-education-research.com
Carbon electrons will be bigger than chlorine electrons Science Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic table, also. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven. chlorine has 7 electrons in its valence shell. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is the central atom.. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From www.numerade.com
SOLVED 'Hello, can someone please help me, Thank You The diagram below Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. chlorine has 7 electrons in its valence shell. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is the central atom. chlorine, with 7 valence electrons, most readily bonds with elements from group. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With chlorine has 7 electrons in its valence shell. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven. To meet the octet rule, it must either gain one electron or lose seven. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. chlorine, with 7 valence. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From www.slideserve.com
PPT Valence Electrons PowerPoint Presentation, free download ID5581383 Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven. To meet the octet rule, it must either gain one electron or lose seven. this means that calcium #s^2# has 2 valence electrons it readily gives away in order to seek the stability of the octet. 93 rows you may assume the valences of the. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From www.numerade.com
SOLVED 'Select the correct answer. How many valence electrons does the Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With chlorine has 7 electrons in its valence shell. this means that calcium #s^2# has 2 valence electrons it readily gives away in order to seek the stability of the octet. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven. To meet the octet rule, it must either gain one electron or lose seven. . Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From dxosvaiso.blob.core.windows.net
Chlorine Element Number Of Isotopes at Calvin Petillo blog Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With this means that calcium #s^2# has 2 valence electrons it readily gives away in order to seek the stability of the octet. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is. the. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From ladergadget.weebly.com
Valence electrons periodic table ladergadget Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With this means that calcium #s^2# has 2 valence electrons it readily gives away in order to seek the stability of the octet. chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic table, also. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From www.numerade.com
SOLVED Use the following examples to answer the questions below. cie Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is. To meet the octet rule, it must either gain one electron or lose seven. nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is the. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From valenceelectrons.com
How Many Valence Electrons Does PCl3 Have? Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven. To meet the octet rule, it must either gain one electron or lose seven. this means that calcium #s^2# has 2 valence electrons it readily gives away in order to seek the stability of the octet. chlorine has 7 electrons in its valence shell. . Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From chemistry98.blogspot.com
Chem Easy Formation of covalent bond in chlorine molecule Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven. this means that calcium #s^2# has 2 valence electrons it readily gives away in order to seek the stability of the octet. chlorine has 7 electrons in its valence shell. To meet the octet rule, it must either gain one electron or lose seven. . Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.
From www.sliderbase.com
Valence Electrons Presentation Chemistry Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With To meet the octet rule, it must either gain one electron or lose seven. the 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven. chlorine, with 7 valence electrons, most readily bonds with elements from group 1a of the periodic table, also. this means that calcium #s^2# has 2 valence electrons it readily gives away. Chlorine Has 7 Valence Electrons. Which Group Will Chlorine Most Likely Bond With.