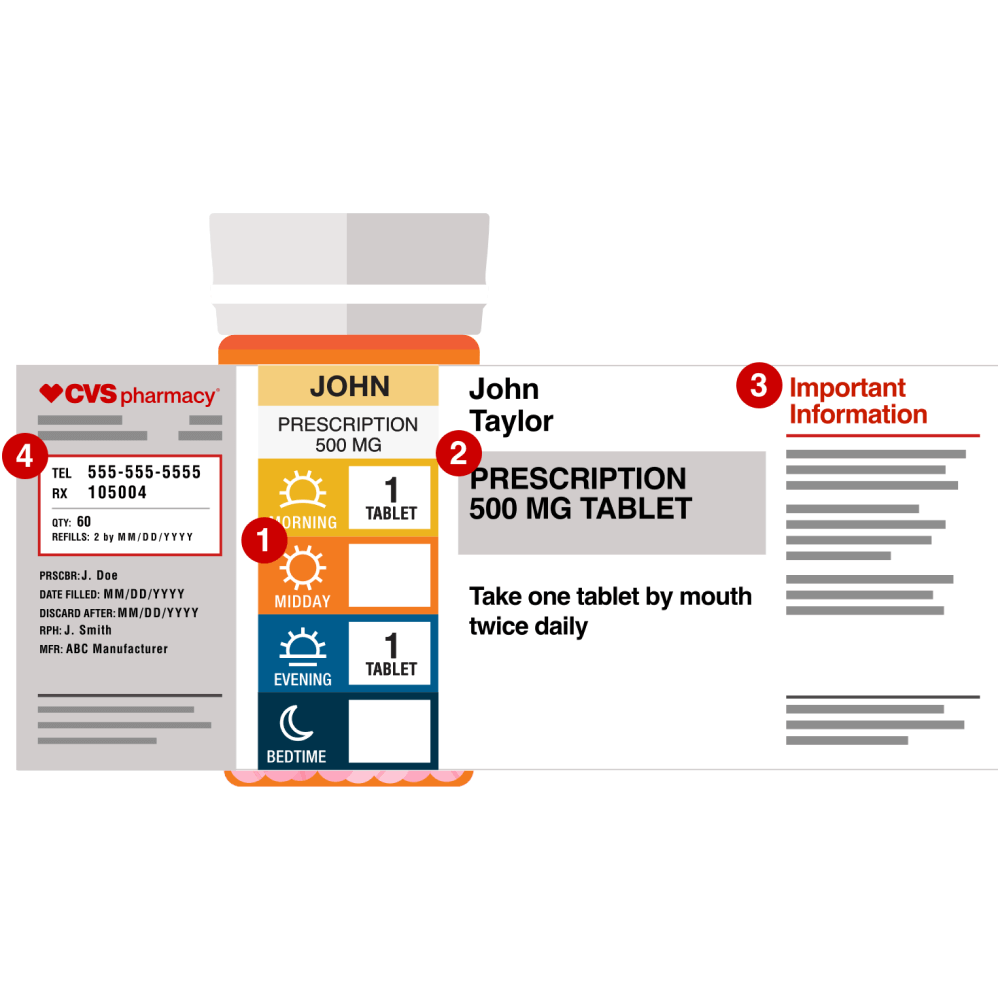
What Is Legally Required To Be Included On A Medication Label . The following is legally required to be on the prescription label, except the: Proposed by the drug company, reviewed by the fda, and. The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This is to ensure that the. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: A specific warning relating to a use not provided for under the “indications and usage” section of the labeling may be required by the food and. Food and drug administration (fda) strictly enforces drug labeling and instructs manufacturers on what information must appear on a label. (1) the labeling must contain a.
from www.vrogue.co
Proposed by the drug company, reviewed by the fda, and. Food and drug administration (fda) strictly enforces drug labeling and instructs manufacturers on what information must appear on a label. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This is to ensure that the. The following is legally required to be on the prescription label, except the: The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented. (1) the labeling must contain a. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: A specific warning relating to a use not provided for under the “indications and usage” section of the labeling may be required by the food and.
Prescription Bottle Label Template Download Template vrogue.co
What Is Legally Required To Be Included On A Medication Label Food and drug administration (fda) strictly enforces drug labeling and instructs manufacturers on what information must appear on a label. This is to ensure that the. The following is legally required to be on the prescription label, except the: Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Proposed by the drug company, reviewed by the fda, and. Food and drug administration (fda) strictly enforces drug labeling and instructs manufacturers on what information must appear on a label. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: (1) the labeling must contain a. A specific warning relating to a use not provided for under the “indications and usage” section of the labeling may be required by the food and. The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented.
From mediqueproducts.com
Medique Products The Brands That Work What Is Legally Required To Be Included On A Medication Label Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented. Food and drug administration (fda) strictly enforces drug. What Is Legally Required To Be Included On A Medication Label.
From www.fda.gov.ph
Draft for Comments Guidelines on Labeling Requirements of Drug What Is Legally Required To Be Included On A Medication Label Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. (1) the labeling must contain a. Proposed by the drug company, reviewed by the fda, and. The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented. Prescription drug labeling described in §. What Is Legally Required To Be Included On A Medication Label.
From hub.arkansasbluecross.com
Deciphering Your Prescription Medication Label Blueprint What Is Legally Required To Be Included On A Medication Label Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Food and drug administration (fda) strictly enforces drug labeling and instructs manufacturers on what information must appear on a label. The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented. Proposed by the drug company, reviewed by. What Is Legally Required To Be Included On A Medication Label.
From www.slideshare.net
Reading Medication Labels What Is Legally Required To Be Included On A Medication Label (1) the labeling must contain a. The following is legally required to be on the prescription label, except the: The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented. A specific warning relating to a use not provided for under the “indications and usage” section of the labeling may be required. What Is Legally Required To Be Included On A Medication Label.
From homedesignideas.help
Is It A Legal Requirement To Have A Disabled Toilet BEST HOME DESIGN What Is Legally Required To Be Included On A Medication Label Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented. A specific warning relating to a use not provided for under the “indications and usage” section of the labeling may be required. What Is Legally Required To Be Included On A Medication Label.
From www.grc-health.com
Investigational Medicinal Product labelling an overview — GRCHealth What Is Legally Required To Be Included On A Medication Label (1) the labeling must contain a. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: The following is legally required to be on the prescription label, except the: Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Proposed by the drug company, reviewed by the. What Is Legally Required To Be Included On A Medication Label.
From www.etsy.com
Prescription Label Template Editable Horizontal RX Bottle Label What Is Legally Required To Be Included On A Medication Label Proposed by the drug company, reviewed by the fda, and. The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented. Food and drug administration (fda) strictly enforces drug labeling and instructs manufacturers on what information must appear on a label. Human prescription drug labeling (1) contains a summary of the essential. What Is Legally Required To Be Included On A Medication Label.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels What Is Legally Required To Be Included On A Medication Label Food and drug administration (fda) strictly enforces drug labeling and instructs manufacturers on what information must appear on a label. Proposed by the drug company, reviewed by the fda, and. This is to ensure that the. The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented. Prescription drug labeling described in. What Is Legally Required To Be Included On A Medication Label.
From www.fda.gov
OTC Drug Facts Label FDA What Is Legally Required To Be Included On A Medication Label Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented. (1) the labeling must contain a. The following is legally required to be on the prescription label, except the: A specific warning relating to a use not. What Is Legally Required To Be Included On A Medication Label.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels What Is Legally Required To Be Included On A Medication Label The following is legally required to be on the prescription label, except the: Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Food and drug administration (fda) strictly enforces drug labeling and instructs manufacturers on what. What Is Legally Required To Be Included On A Medication Label.
From www.umc.edu
Medication labels University of Mississippi Medical Center What Is Legally Required To Be Included On A Medication Label The following is legally required to be on the prescription label, except the: A specific warning relating to a use not provided for under the “indications and usage” section of the labeling may be required by the food and. This is to ensure that the. The requirements in this section apply only to prescription drug products described in § 201.56(b)(1). What Is Legally Required To Be Included On A Medication Label.
From www.etsy.com
Prescription Label Template Editable RX Bottle Label Template Party What Is Legally Required To Be Included On A Medication Label Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Proposed by the drug company, reviewed by the fda, and. The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented. The following is legally required to be on the prescription label, except. What Is Legally Required To Be Included On A Medication Label.
From www.express-scripts.com
Understand your medication label Express Scripts® Pharmacy What Is Legally Required To Be Included On A Medication Label Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. The following is legally required to be on the prescription label, except the: A specific warning relating to a use not provided for under the “indications and. What Is Legally Required To Be Included On A Medication Label.
From www.osmosis.org
Reading Medication Labels Osmosis Video Library What Is Legally Required To Be Included On A Medication Label Food and drug administration (fda) strictly enforces drug labeling and instructs manufacturers on what information must appear on a label. This is to ensure that the. A specific warning relating to a use not provided for under the “indications and usage” section of the labeling may be required by the food and. Proposed by the drug company, reviewed by the. What Is Legally Required To Be Included On A Medication Label.
From www.3zdental.ca
Medication Added Label 3Z Dental What Is Legally Required To Be Included On A Medication Label (1) the labeling must contain a. The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented. Food and drug administration (fda) strictly enforces drug labeling and instructs manufacturers on what information must appear on a label. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Human. What Is Legally Required To Be Included On A Medication Label.
From www.etsy.com
Pill Bottle Labels Printable Editable Prescription Label Etsy What Is Legally Required To Be Included On A Medication Label Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: A specific warning relating to a use not provided for under the “indications and usage” section of the labeling may be required by the food and. Food and drug administration (fda) strictly enforces drug labeling and instructs manufacturers on what information must appear on a label.. What Is Legally Required To Be Included On A Medication Label.
From www.etsy.com
Pill Bottle Label RX Bottle Label Nurse Party Favors Etsy What Is Legally Required To Be Included On A Medication Label Food and drug administration (fda) strictly enforces drug labeling and instructs manufacturers on what information must appear on a label. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: (1) the labeling must contain a. A. What Is Legally Required To Be Included On A Medication Label.
From brennad-images.blogspot.com
Printable Prescription Warning Labels / Ers Solutions Pharmacy What Is Legally Required To Be Included On A Medication Label This is to ensure that the. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Proposed by the drug company, reviewed by the fda, and. Food and drug administration (fda) strictly enforces drug labeling and instructs manufacturers on what information must appear on a label. Human prescription drug labeling (1) contains a summary of the. What Is Legally Required To Be Included On A Medication Label.
From www.fda.gov
The OvertheCounter Medicine Label Take a Look FDA What Is Legally Required To Be Included On A Medication Label A specific warning relating to a use not provided for under the “indications and usage” section of the labeling may be required by the food and. The following is legally required to be on the prescription label, except the: Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: This is to ensure that the. Food. What Is Legally Required To Be Included On A Medication Label.
From www.umc.edu
Medication labels University of Mississippi Medical Center What Is Legally Required To Be Included On A Medication Label Food and drug administration (fda) strictly enforces drug labeling and instructs manufacturers on what information must appear on a label. Proposed by the drug company, reviewed by the fda, and. The following is legally required to be on the prescription label, except the: A specific warning relating to a use not provided for under the “indications and usage” section of. What Is Legally Required To Be Included On A Medication Label.
From www.youtube.com
How to read a medication label YouTube What Is Legally Required To Be Included On A Medication Label Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: The following is legally required to be on the prescription label, except the: A specific warning relating to a use not provided for under the “indications and. What Is Legally Required To Be Included On A Medication Label.
From aplmed.com
Prescription Medication Label 2 Aplmed Academy What Is Legally Required To Be Included On A Medication Label Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: (1) the labeling must contain a. This is to ensure that the. A specific warning relating to a use not provided for under the “indications and usage”. What Is Legally Required To Be Included On A Medication Label.
From healthyheels.org
Medication Label Literacy UNC Healthy Heels What Is Legally Required To Be Included On A Medication Label This is to ensure that the. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. A specific warning relating to a use not provided for under the “indications and usage” section of the labeling may be required by the food and. (1) the labeling must contain a. The requirements in. What Is Legally Required To Be Included On A Medication Label.
From scielo.isciii.es
Improving pediatric liquid medication labeling of the hospital What Is Legally Required To Be Included On A Medication Label This is to ensure that the. The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented. Proposed by the drug company, reviewed by the fda, and. A specific warning relating to a use not provided for under the “indications and usage” section of the labeling may be required by the food. What Is Legally Required To Be Included On A Medication Label.
From mavink.com
Sample Medication Labels What Is Legally Required To Be Included On A Medication Label This is to ensure that the. Proposed by the drug company, reviewed by the fda, and. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: (1) the labeling must contain a. A specific warning relating to. What Is Legally Required To Be Included On A Medication Label.
From www.distinctivemedical.com
Medication Added Label Distinctive Medical What Is Legally Required To Be Included On A Medication Label Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented. (1) the labeling must contain a. Food and drug administration (fda) strictly enforces drug labeling and instructs manufacturers on what information must appear on a label. Human. What Is Legally Required To Be Included On A Medication Label.
From rossendaleharriers.co.uk
Some Ideas on Medication Safety And Poison Prevention For Older Adults What Is Legally Required To Be Included On A Medication Label Food and drug administration (fda) strictly enforces drug labeling and instructs manufacturers on what information must appear on a label. A specific warning relating to a use not provided for under the “indications and usage” section of the labeling may be required by the food and. This is to ensure that the. The following is legally required to be on. What Is Legally Required To Be Included On A Medication Label.
From my.clevelandclinic.org
How To Read A Prescription Label A Guide Cleveland Clinic What Is Legally Required To Be Included On A Medication Label The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Food and drug administration (fda) strictly enforces drug labeling and instructs manufacturers on what information must appear on a label. Prescription drug. What Is Legally Required To Be Included On A Medication Label.
From emmainternational.com
Discovering FDALabel Your GoTo Labelling Tool What Is Legally Required To Be Included On A Medication Label Proposed by the drug company, reviewed by the fda, and. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: The following is legally required to be on the prescription label, except the: Food and drug administration (fda) strictly enforces drug labeling and instructs manufacturers on what information must appear on a label. This is to. What Is Legally Required To Be Included On A Medication Label.
From healthyheels.org
medication UNC Healthy Heels What Is Legally Required To Be Included On A Medication Label A specific warning relating to a use not provided for under the “indications and usage” section of the labeling may be required by the food and. The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented. The following is legally required to be on the prescription label, except the: Proposed by. What Is Legally Required To Be Included On A Medication Label.
From www.alamy.com
The label on a bottle of penicillin antibiotic medicine, UK Stock Photo What Is Legally Required To Be Included On A Medication Label Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Proposed by the drug company, reviewed by the fda, and. (1) the labeling must contain a. Food and drug administration (fda) strictly enforces drug labeling and instructs manufacturers on what information must appear on a label. Human prescription drug labeling (1) contains a summary of the. What Is Legally Required To Be Included On A Medication Label.
From vivafda.com
FDA Drug Labeling and Ingredient Requirement Viva FDA U.S. FDA What Is Legally Required To Be Included On A Medication Label The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: This is to ensure that the. Proposed by the drug company, reviewed by the fda, and. Food and drug administration (fda) strictly enforces drug labeling and instructs. What Is Legally Required To Be Included On A Medication Label.
From etactics.com
Prescription Label Design Why It Matters and Effective Examples — Etactics What Is Legally Required To Be Included On A Medication Label Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Proposed by the drug company, reviewed by the fda, and. (1) the labeling must contain a. A specific warning relating to a use not provided for under the “indications and usage” section of the labeling may be required by the food and. The following is legally. What Is Legally Required To Be Included On A Medication Label.
From rxoutreach.org
Education Understanding Prescription Medication Labels Rx Outreach What Is Legally Required To Be Included On A Medication Label (1) the labeling must contain a. A specific warning relating to a use not provided for under the “indications and usage” section of the labeling may be required by the food and. The following is legally required to be on the prescription label, except the: Proposed by the drug company, reviewed by the fda, and. Human prescription drug labeling (1). What Is Legally Required To Be Included On A Medication Label.
From www.vrogue.co
Prescription Bottle Label Template Download Template vrogue.co What Is Legally Required To Be Included On A Medication Label (1) the labeling must contain a. The following is legally required to be on the prescription label, except the: This is to ensure that the. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: The requirements. What Is Legally Required To Be Included On A Medication Label.