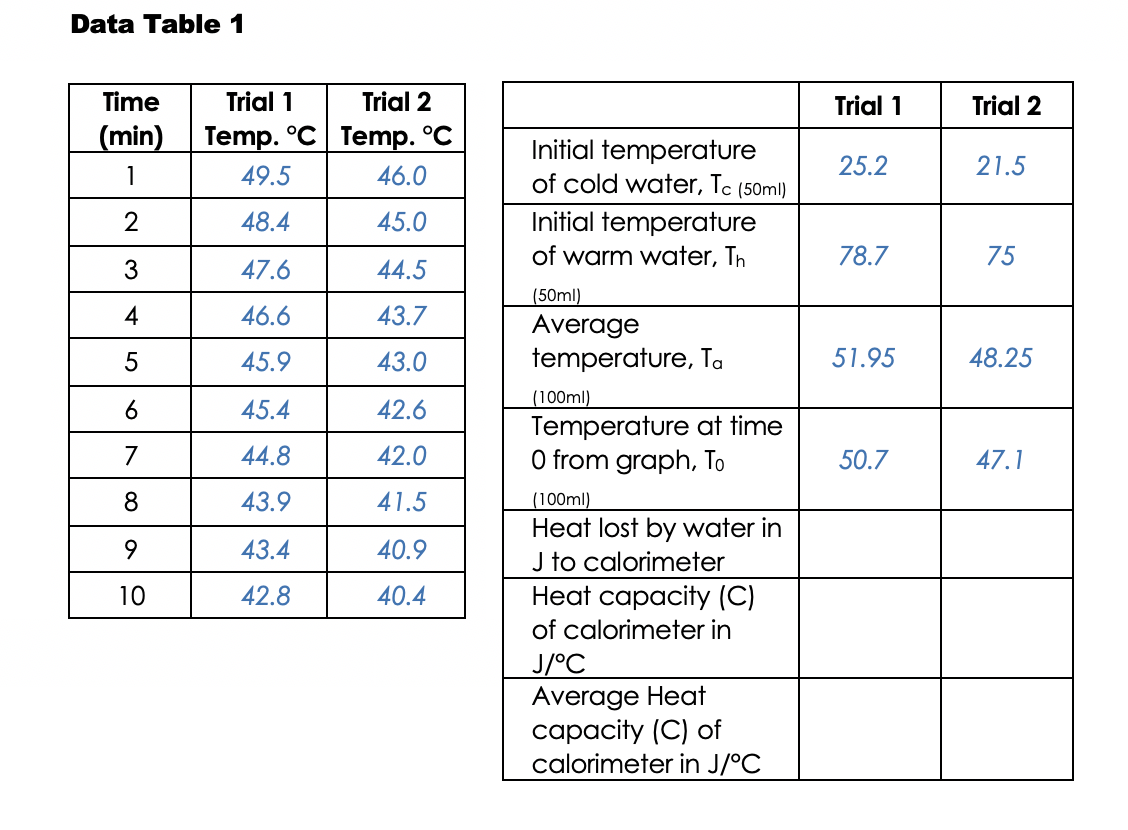
How To Calculate Heat Lost By Calorimeter . It can analyze the heat. \delta q = m c \delta t δq = mcδt. apply the first law of thermodynamics to calorimetry. the calorimetry calculator can help you solve complex calorimetry problems. heat transfer restores thermal equilibrium once the water and pan are in contact; Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. (the specific heat capacity of water is 4.184 j g¯ 1 °c¯ 1). the amount of heat gained or lost can be calculated by the following equation. calculate the heat capacity of the calorimeter. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. It stops once thermal equilibrium between. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the.
from www.chegg.com
(the specific heat capacity of water is 4.184 j g¯ 1 °c¯ 1). the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. It can analyze the heat. the amount of heat gained or lost can be calculated by the following equation. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. heat transfer restores thermal equilibrium once the water and pan are in contact; It stops once thermal equilibrium between. \delta q = m c \delta t δq = mcδt. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. apply the first law of thermodynamics to calorimetry.
Solved 1. Heat Lost by Water in J to calorimeter. 2.
How To Calculate Heat Lost By Calorimeter the amount of heat gained or lost can be calculated by the following equation. the amount of heat gained or lost can be calculated by the following equation. calculate the heat capacity of the calorimeter. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. (the specific heat capacity of water is 4.184 j g¯ 1 °c¯ 1). the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. apply the first law of thermodynamics to calorimetry. It can analyze the heat. the calorimetry calculator can help you solve complex calorimetry problems. It stops once thermal equilibrium between. \delta q = m c \delta t δq = mcδt. heat transfer restores thermal equilibrium once the water and pan are in contact; the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the.
From studyadvertiser.z21.web.core.windows.net
How To Use A Calorimeter Stepbystep How To Calculate Heat Lost By Calorimeter apply the first law of thermodynamics to calorimetry. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. calculate the heat capacity of the calorimeter. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is. How To Calculate Heat Lost By Calorimeter.
From learningschoolsmmnifl.z4.web.core.windows.net
Heat Of Reaction Chemistry How To Calculate Heat Lost By Calorimeter the amount of heat gained or lost can be calculated by the following equation. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. It can analyze the heat. the temperature increase is measured and, along with the known heat capacity of. How To Calculate Heat Lost By Calorimeter.
From lessonlistleis.z1.web.core.windows.net
Calculating Heat And Specific Heat Worksheets How To Calculate Heat Lost By Calorimeter calculate the heat capacity of the calorimeter. (the specific heat capacity of water is 4.184 j g¯ 1 °c¯ 1). the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. the heat capacity of the calorimeter or of the reaction mixture may. How To Calculate Heat Lost By Calorimeter.
From worksheetmediadwaum.z14.web.core.windows.net
How To Solve Calorimetry Problems How To Calculate Heat Lost By Calorimeter calculate the heat capacity of the calorimeter. apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. (the specific heat capacity of water is 4.184 j g¯ 1 °c¯ 1). the heat capacity of the calorimeter or of the reaction mixture may be. How To Calculate Heat Lost By Calorimeter.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube How To Calculate Heat Lost By Calorimeter apply the first law of thermodynamics to calorimetry. It can analyze the heat. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. calculate the heat capacity of the calorimeter. It stops once thermal equilibrium between. Compare heat flow from hot to. How To Calculate Heat Lost By Calorimeter.
From www.slideserve.com
PPT Section 7 HEAT LOSS CALCULATIONS PowerPoint Presentation, free download ID4656215 How To Calculate Heat Lost By Calorimeter It can analyze the heat. the calorimetry calculator can help you solve complex calorimetry problems. apply the first law of thermodynamics to calorimetry. calculate the heat capacity of the calorimeter. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. the temperature increase is measured and, along with the known. How To Calculate Heat Lost By Calorimeter.
From learningcampusscarf.z13.web.core.windows.net
How To Calculate Heat Of Reaction Calorimetry How To Calculate Heat Lost By Calorimeter \delta q = m c \delta t δq = mcδt. apply the first law of thermodynamics to calorimetry. the amount of heat gained or lost can be calculated by the following equation. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the.. How To Calculate Heat Lost By Calorimeter.
From www.youtube.com
AP Chemistry Thermochemistry I Part 4 Constant Pressure Calorimetry YouTube How To Calculate Heat Lost By Calorimeter the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. the heat capacity of the calorimeter or of the reaction mixture may. How To Calculate Heat Lost By Calorimeter.
From www.slideserve.com
PPT CALORIMETRY PowerPoint Presentation, free download ID6454425 How To Calculate Heat Lost By Calorimeter It can analyze the heat. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. apply the first law of thermodynamics to calorimetry. the calorimetry calculator can help you solve complex calorimetry problems. the heat capacity of the calorimeter or of the reaction mixture may be. How To Calculate Heat Lost By Calorimeter.
From www.youtube.com
How to Calculate Enthalpy Change Using a Calorimeter YouTube How To Calculate Heat Lost By Calorimeter the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. heat transfer restores thermal equilibrium once the water and pan are in contact; It stops once thermal equilibrium between. It can analyze. How To Calculate Heat Lost By Calorimeter.
From learningcampusscarf.z13.web.core.windows.net
How To Calculate Heat Of Reaction Calorimetry How To Calculate Heat Lost By Calorimeter apply the first law of thermodynamics to calorimetry. the amount of heat gained or lost can be calculated by the following equation. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. It can analyze the heat. Compare heat flow from hot. How To Calculate Heat Lost By Calorimeter.
From lessonlibrarystiletto.z13.web.core.windows.net
How To Calculate Heat Lost How To Calculate Heat Lost By Calorimeter the amount of heat gained or lost can be calculated by the following equation. (the specific heat capacity of water is 4.184 j g¯ 1 °c¯ 1). It stops once thermal equilibrium between. \delta q = m c \delta t δq = mcδt. It can analyze the heat. the heat capacity of the calorimeter or of the reaction. How To Calculate Heat Lost By Calorimeter.
From www.chegg.com
Solved 1. Heat Lost by Water in J to calorimeter. 2. How To Calculate Heat Lost By Calorimeter the calorimetry calculator can help you solve complex calorimetry problems. the amount of heat gained or lost can be calculated by the following equation. calculate the heat capacity of the calorimeter. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. It stops once thermal equilibrium. How To Calculate Heat Lost By Calorimeter.
From revisionug.com
What is Calorimetry? How To Calculate Heat Lost By Calorimeter the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. It stops once thermal equilibrium between. \delta q = m c \delta t δq = mcδt. It can analyze the heat. calculate the heat capacity of the calorimeter. heat transfer restores thermal. How To Calculate Heat Lost By Calorimeter.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download ID4784803 How To Calculate Heat Lost By Calorimeter It stops once thermal equilibrium between. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. the amount of heat gained or. How To Calculate Heat Lost By Calorimeter.
From www.wikihow.com
How to Calculate Heat Capacity 8 Steps (with Pictures) wikiHow How To Calculate Heat Lost By Calorimeter the calorimetry calculator can help you solve complex calorimetry problems. heat transfer restores thermal equilibrium once the water and pan are in contact; the amount of heat gained or lost can be calculated by the following equation. apply the first law of thermodynamics to calorimetry. the heat capacity of the calorimeter or of the reaction. How To Calculate Heat Lost By Calorimeter.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube How To Calculate Heat Lost By Calorimeter (the specific heat capacity of water is 4.184 j g¯ 1 °c¯ 1). It stops once thermal equilibrium between. It can analyze the heat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. apply the first law of thermodynamics to calorimetry. heat transfer restores thermal equilibrium once the water and pan. How To Calculate Heat Lost By Calorimeter.
From www.chegg.com
Solved Calculations I. Heat Capacity of the Calorimeter The How To Calculate Heat Lost By Calorimeter \delta q = m c \delta t δq = mcδt. (the specific heat capacity of water is 4.184 j g¯ 1 °c¯ 1). calculate the heat capacity of the calorimeter. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. It can analyze. How To Calculate Heat Lost By Calorimeter.
From www.thetechedvocate.org
How to calculate heat gained by water The Tech Edvocate How To Calculate Heat Lost By Calorimeter (the specific heat capacity of water is 4.184 j g¯ 1 °c¯ 1). heat transfer restores thermal equilibrium once the water and pan are in contact; It can analyze the heat. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. Compare heat flow from hot to cold. How To Calculate Heat Lost By Calorimeter.
From haipernews.com
How To Calculate Heat Capacity Of A Calorimeter Haiper How To Calculate Heat Lost By Calorimeter calculate the heat capacity of the calorimeter. It can analyze the heat. (the specific heat capacity of water is 4.184 j g¯ 1 °c¯ 1). the amount of heat gained or lost can be calculated by the following equation. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. heat transfer. How To Calculate Heat Lost By Calorimeter.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation ID6591088 How To Calculate Heat Lost By Calorimeter heat transfer restores thermal equilibrium once the water and pan are in contact; It stops once thermal equilibrium between. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. It can analyze the heat. the heat capacity of the calorimeter or of the reaction mixture may be. How To Calculate Heat Lost By Calorimeter.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID6133898 How To Calculate Heat Lost By Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. apply the first law of thermodynamics to calorimetry. heat transfer restores thermal equilibrium once the water and pan are in contact; the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the.. How To Calculate Heat Lost By Calorimeter.
From www.youtube.com
How to find calorimeter constant YouTube How To Calculate Heat Lost By Calorimeter the calorimetry calculator can help you solve complex calorimetry problems. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. heat transfer restores thermal equilibrium once the water and pan are in contact; It can analyze the heat. \delta q = m c \delta t δq =. How To Calculate Heat Lost By Calorimeter.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper How To Calculate Heat Lost By Calorimeter the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. the amount of heat gained or lost can be calculated by the following equation. It can analyze the heat. the calorimetry calculator can help you solve complex calorimetry problems. Compare heat flow from hot to cold objects. How To Calculate Heat Lost By Calorimeter.
From cider.uoregon.edu
Calorimetry Heat Exchange Hot Metal in Cold Water Real and Computer Simulation CIDER How To Calculate Heat Lost By Calorimeter the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. It can analyze the heat. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. It stops once thermal equilibrium between. heat. How To Calculate Heat Lost By Calorimeter.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog How To Calculate Heat Lost By Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. (the specific heat capacity of water is 4.184 j g¯ 1 °c¯ 1). It stops once thermal equilibrium between. the heat capacity. How To Calculate Heat Lost By Calorimeter.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube How To Calculate Heat Lost By Calorimeter It can analyze the heat. the amount of heat gained or lost can be calculated by the following equation. (the specific heat capacity of water is 4.184 j g¯ 1 °c¯ 1). the calorimetry calculator can help you solve complex calorimetry problems. the temperature increase is measured and, along with the known heat capacity of the calorimeter,. How To Calculate Heat Lost By Calorimeter.
From quizmischances.z4.web.core.windows.net
How To Calculate Calorimetry How To Calculate Heat Lost By Calorimeter the amount of heat gained or lost can be calculated by the following equation. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. \delta q =. How To Calculate Heat Lost By Calorimeter.
From jsmithmoore.com
Heat loss calculator chemistry How To Calculate Heat Lost By Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. It can analyze the heat. the amount of heat gained or lost can be calculated by the following equation. apply the first law of thermodynamics to calorimetry. the heat capacity of the calorimeter or of the reaction mixture may be used. How To Calculate Heat Lost By Calorimeter.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to Calculate the Heat How To Calculate Heat Lost By Calorimeter the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. the amount of heat gained or lost can be calculated by the following equation. heat transfer restores thermal equilibrium once the water and pan are in contact; It stops once thermal equilibrium between. apply the first. How To Calculate Heat Lost By Calorimeter.
From www.chegg.com
Solved Calculate Heat capacity of the calorimeter (J/ ºC) , How To Calculate Heat Lost By Calorimeter (the specific heat capacity of water is 4.184 j g¯ 1 °c¯ 1). It stops once thermal equilibrium between. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. \delta q = m c \delta t δq = mcδt. apply the first law. How To Calculate Heat Lost By Calorimeter.
From quizmischances.z4.web.core.windows.net
How To Calculate Calorimetry How To Calculate Heat Lost By Calorimeter the calorimetry calculator can help you solve complex calorimetry problems. the amount of heat gained or lost can be calculated by the following equation. It can analyze the heat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. calculate the heat capacity of the calorimeter. \delta q = m c. How To Calculate Heat Lost By Calorimeter.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 How To Calculate Heat Lost By Calorimeter It stops once thermal equilibrium between. \delta q = m c \delta t δq = mcδt. apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. calculate the heat capacity of the calorimeter. the temperature increase is measured and, along with the known. How To Calculate Heat Lost By Calorimeter.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube How To Calculate Heat Lost By Calorimeter the calorimetry calculator can help you solve complex calorimetry problems. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or. How To Calculate Heat Lost By Calorimeter.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID6655927 How To Calculate Heat Lost By Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. \delta q = m c \delta t δq = mcδt. apply the first law of thermodynamics to calorimetry. calculate the heat capacity of the calorimeter. heat transfer restores thermal equilibrium once the water and pan are in contact; the calorimetry. How To Calculate Heat Lost By Calorimeter.