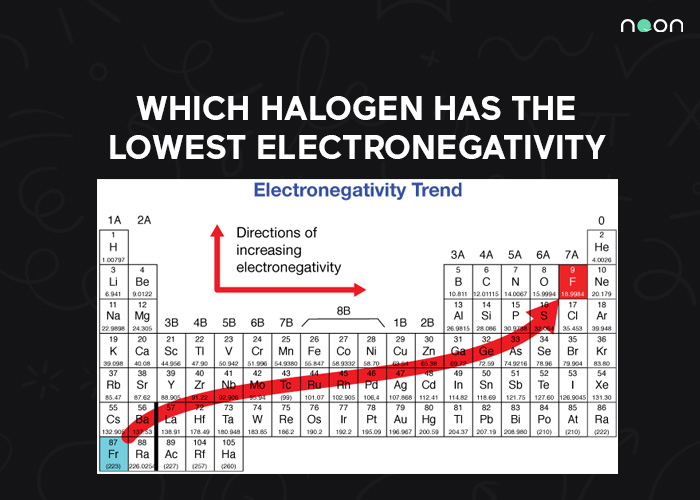
Chlorine Has A Higher Electronegativity . Consider sodium at the beginning of period 3 and chlorine at the end. Because sr lies far to the left of. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. cl 2 must be nonpolar because the electronegativity difference (δχ) is zero; why does electronegativity increase across a period? a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). Hence the two chlorine atoms share the. the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. the chlorine atom has a higher electronegativity than the hydrogen atom, so the.
from www.learnatnoon.com
the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). the chlorine atom has a higher electronegativity than the hydrogen atom, so the. Hence the two chlorine atoms share the. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. cl 2 must be nonpolar because the electronegativity difference (δχ) is zero; Consider sodium at the beginning of period 3 and chlorine at the end. Because sr lies far to the left of. why does electronegativity increase across a period?
Which Halogen Has The Lowest Electronegativity? Noon Academy
Chlorine Has A Higher Electronegativity the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. why does electronegativity increase across a period? Hence the two chlorine atoms share the. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). Consider sodium at the beginning of period 3 and chlorine at the end. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. cl 2 must be nonpolar because the electronegativity difference (δχ) is zero; the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. the chlorine atom has a higher electronegativity than the hydrogen atom, so the. Because sr lies far to the left of.
From www.learnatnoon.com
Which Halogen Has The Lowest Electronegativity? Noon Academy Chlorine Has A Higher Electronegativity 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Consider sodium at the beginning of period 3 and chlorine at the end. why does electronegativity increase across a period? the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. Chlorine Has A Higher Electronegativity.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Chlorine Has A Higher Electronegativity the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. why does electronegativity increase across a period? the chlorine atom has a higher electronegativity than the hydrogen atom, so the. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic. Chlorine Has A Higher Electronegativity.
From mavink.com
Element Electronegativity Chart Chlorine Has A Higher Electronegativity a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). Consider sodium at the beginning of period 3 and chlorine at the end. why does electronegativity increase across a period? the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. . Chlorine Has A Higher Electronegativity.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Has A Higher Electronegativity the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. Consider sodium at the beginning of period 3 and chlorine at the end. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). Because sr lies far to the left of. . Chlorine Has A Higher Electronegativity.
From periodictable.me
Electronegativity Chlorine Has A Higher Electronegativity Consider sodium at the beginning of period 3 and chlorine at the end. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). the chlorine atom has a higher electronegativity than the hydrogen atom, so the. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and. Chlorine Has A Higher Electronegativity.
From www.youtube.com
Electronegativity, Basic Introduction, Periodic Trends Which Element Chlorine Has A Higher Electronegativity Consider sodium at the beginning of period 3 and chlorine at the end. cl 2 must be nonpolar because the electronegativity difference (δχ) is zero; why does electronegativity increase across a period? 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the chlorine atom has a higher electronegativity. Chlorine Has A Higher Electronegativity.
From chemistry.stackexchange.com
periodic trends If fluorine has a lower electron affinity than Chlorine Has A Higher Electronegativity 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Because sr lies far to the left of. cl 2 must be nonpolar because the electronegativity difference (δχ) is zero; a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). Hence the two chlorine atoms. Chlorine Has A Higher Electronegativity.
From socratic.org
What would cause an atom to have a low electronegativity value? Socratic Chlorine Has A Higher Electronegativity Hence the two chlorine atoms share the. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). Because sr lies far to the left of. the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. 119 rows electronegativity is. Chlorine Has A Higher Electronegativity.
From www.meritnation.com
pls explain why fluorine has less negative electron gain enthalpy than Chlorine Has A Higher Electronegativity the chlorine atom has a higher electronegativity than the hydrogen atom, so the. why does electronegativity increase across a period? 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Because sr lies far to the left of. a electronegativity increases from lower left to upper right in the. Chlorine Has A Higher Electronegativity.
From blog.naver.com
Electronegativity table, 전기음성도 표 네이버 블로그 Chlorine Has A Higher Electronegativity Because sr lies far to the left of. Consider sodium at the beginning of period 3 and chlorine at the end. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). Hence the two chlorine atoms share the. why does electronegativity increase across a period? 119 rows electronegativity is used to predict. Chlorine Has A Higher Electronegativity.
From www.coursehero.com
[Solved] . 1. Chlorine has a higher electronegativity than sulfur. T Chlorine Has A Higher Electronegativity a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). Consider sodium at the beginning of period 3 and chlorine at the end. the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. Because sr lies far to the left. Chlorine Has A Higher Electronegativity.
From brainly.com
which atom in each pair that has the greater electronegativity. a. Ca Chlorine Has A Higher Electronegativity the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. Chlorine Has A Higher Electronegativity.
From dxoddvwxb.blob.core.windows.net
Magnesium Chloride Electronegativity at Herman blog Chlorine Has A Higher Electronegativity Because sr lies far to the left of. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Hence the two chlorine atoms share the. cl 2 must be nonpolar because the electronegativity difference (δχ) is zero; why does electronegativity increase across a period? a electronegativity increases from lower. Chlorine Has A Higher Electronegativity.
From socratic.org
Question f6b1f Socratic Chlorine Has A Higher Electronegativity the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. Hence the two chlorine atoms share the. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the chlorine atom has a higher electronegativity than the hydrogen. Chlorine Has A Higher Electronegativity.
From www.bigstockphoto.com
Electronegativity Image & Photo (Free Trial) Bigstock Chlorine Has A Higher Electronegativity 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Hence the two chlorine atoms share the. cl 2 must be nonpolar because the electronegativity difference (δχ) is zero; the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on. Chlorine Has A Higher Electronegativity.
From byjus.com
why fluorine is more electronegative than oxygen. Chlorine Has A Higher Electronegativity why does electronegativity increase across a period? a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). Because sr lies far to the left of. Hence the two chlorine atoms share the. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Consider sodium at. Chlorine Has A Higher Electronegativity.
From surfguppy.com
Electronegativity Bond Scale Surfguppy Chemistry made easy for Chlorine Has A Higher Electronegativity Hence the two chlorine atoms share the. the chlorine atom has a higher electronegativity than the hydrogen atom, so the. Because sr lies far to the left of. Consider sodium at the beginning of period 3 and chlorine at the end. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent.. Chlorine Has A Higher Electronegativity.
From secrets-of-periodic-table.blogspot.com
PERIODIC TABLE ELECTRONEGATIVITY NOBLE GASES PAULING Chlorine Has A Higher Electronegativity Because sr lies far to the left of. the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. a electronegativity increases from lower left to upper right. Chlorine Has A Higher Electronegativity.
From www.numerade.com
SOLVED Choose all the statements that are correct. (1) Like atomic Chlorine Has A Higher Electronegativity 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. why does electronegativity increase across a period? the chlorine atom has a higher electronegativity than the hydrogen atom, so the. Because sr lies far to the left of. the two chlorine atoms share the pair of electrons in the. Chlorine Has A Higher Electronegativity.
From chemistry.stackexchange.com
periodic trends If fluorine has a lower electron affinity than Chlorine Has A Higher Electronegativity Because sr lies far to the left of. why does electronegativity increase across a period? Hence the two chlorine atoms share the. Consider sodium at the beginning of period 3 and chlorine at the end. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. the. Chlorine Has A Higher Electronegativity.
From avopix.com
Electronegativity infographic diagram with Royalty Free Stock Vector Chlorine Has A Higher Electronegativity 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the chlorine atom has a higher electronegativity than the hydrogen atom, so the. Because sr lies far to the left of. the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of. Chlorine Has A Higher Electronegativity.
From www.nanowerk.com
Electronegativity explained Chlorine Has A Higher Electronegativity Consider sodium at the beginning of period 3 and chlorine at the end. Because sr lies far to the left of. the chlorine atom has a higher electronegativity than the hydrogen atom, so the. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. why does electronegativity increase across a. Chlorine Has A Higher Electronegativity.
From www.trentu.ca
PreChemistry Chlorine Has A Higher Electronegativity 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. Hence the two chlorine atoms share the. cl 2 must be nonpolar because the electronegativity difference (δχ) is zero;. Chlorine Has A Higher Electronegativity.
From www.pinterest.co.uk
Illustration about Periodic table of elements with electronegativity Chlorine Has A Higher Electronegativity the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. Because sr lies far to the left of. the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. the chlorine atom has a. Chlorine Has A Higher Electronegativity.
From hxeiqucfl.blob.core.windows.net
Electronegativity Chlorine Higher Than Sulfur at Ronald Bartlett blog Chlorine Has A Higher Electronegativity the chlorine atom has a higher electronegativity than the hydrogen atom, so the. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). cl 2 must be nonpolar because the electronegativity difference (δχ) is zero; Because sr lies far to the left of. Consider sodium at the beginning of period 3 and. Chlorine Has A Higher Electronegativity.
From hxerxizou.blob.core.windows.net
Electronegativity Of Chlorine Is Higher Than Sulphur at Krista Chlorine Has A Higher Electronegativity 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Because sr lies far to the left of. the chlorine atom has a higher electronegativity than the hydrogen atom, so the. cl 2 must be nonpolar because the electronegativity difference (δχ) is zero; a electronegativity increases from lower left. Chlorine Has A Higher Electronegativity.
From dxolxdofb.blob.core.windows.net
Electronegativity Chlorine And Bromine at Tammy Parker blog Chlorine Has A Higher Electronegativity the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. Because sr lies far to the left of. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. why does electronegativity increase across a period? cl. Chlorine Has A Higher Electronegativity.
From blog.naver.com
유기화학 2장. 극성 공유 결합 산과 염기(1) 네이버 블로그 Chlorine Has A Higher Electronegativity the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. the chlorine atom has a higher electronegativity than the hydrogen atom, so the. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). Because sr lies far to the left of.. Chlorine Has A Higher Electronegativity.
From hxeiqucfl.blob.core.windows.net
Electronegativity Chlorine Higher Than Sulfur at Ronald Bartlett blog Chlorine Has A Higher Electronegativity the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the chlorine atom has a higher electronegativity than the hydrogen atom, so the. Hence the two chlorine. Chlorine Has A Higher Electronegativity.
From dxolxdofb.blob.core.windows.net
Electronegativity Chlorine And Bromine at Tammy Parker blog Chlorine Has A Higher Electronegativity Hence the two chlorine atoms share the. the chlorine atom has a higher electronegativity than the hydrogen atom, so the. Consider sodium at the beginning of period 3 and chlorine at the end. Because sr lies far to the left of. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent.. Chlorine Has A Higher Electronegativity.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Chlorine Has A Higher Electronegativity Because sr lies far to the left of. the chlorine atom has a higher electronegativity than the hydrogen atom, so the. Consider sodium at the beginning of period 3 and chlorine at the end. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). the first scale of electronegativity was developed by. Chlorine Has A Higher Electronegativity.
From surfguppy.com
Electronegativity Bond Scale Surfguppy Chemistry made easy for Chlorine Has A Higher Electronegativity a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). Consider sodium at the beginning of period 3 and chlorine at the end. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. cl 2 must be nonpolar because the electronegativity difference (δχ) is zero;. Chlorine Has A Higher Electronegativity.
From dxoddvwxb.blob.core.windows.net
Magnesium Chloride Electronegativity at Herman blog Chlorine Has A Higher Electronegativity the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. Consider sodium at the beginning of period 3 and chlorine at the end. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). why does electronegativity increase across a. Chlorine Has A Higher Electronegativity.
From askfilo.com
3. Although chlorine has same electronegativity as nitrogen but the forme.. Chlorine Has A Higher Electronegativity Consider sodium at the beginning of period 3 and chlorine at the end. cl 2 must be nonpolar because the electronegativity difference (δχ) is zero; the chlorine atom has a higher electronegativity than the hydrogen atom, so the. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. Chlorine Has A Higher Electronegativity.
From exocoyzqy.blob.core.windows.net
Does Chlorine Have A High Electronegativity at Gordon Maxwell blog Chlorine Has A Higher Electronegativity Hence the two chlorine atoms share the. the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. the chlorine atom has a higher electronegativity than the hydrogen atom, so the. the two chlorine atoms share the pair of electrons in the single covalent bond. Chlorine Has A Higher Electronegativity.