Potassium Chlorine Electronegativity Difference . The pauling scale is the. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. the absolute value of the difference in electronegativity (δen) of two bonded atoms provides a rough measure of the polarity to be expected in. you can compare k vs cl on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure,. to predict the polarity of the bonds in cl 2, hcl, and nacl, for example, we look at the electronegativities of the relevant atoms: 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the electronegativity calculator allows you to calculate the type of bond formed between different elements using. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. on moving from left to right in the periodic table, electronegativity increases whereas on moving from top to bottom, the.
from surfguppy.com
119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the absolute value of the difference in electronegativity (δen) of two bonded atoms provides a rough measure of the polarity to be expected in. The pauling scale is the. on moving from left to right in the periodic table, electronegativity increases whereas on moving from top to bottom, the. to predict the polarity of the bonds in cl 2, hcl, and nacl, for example, we look at the electronegativities of the relevant atoms: the electronegativity calculator allows you to calculate the type of bond formed between different elements using. you can compare k vs cl on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure,. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the.
Electronegativity Bond Scale Surfguppy Chemistry made easy visual
Potassium Chlorine Electronegativity Difference to predict the polarity of the bonds in cl 2, hcl, and nacl, for example, we look at the electronegativities of the relevant atoms: The pauling scale is the. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the absolute value of the difference in electronegativity (δen) of two bonded atoms provides a rough measure of the polarity to be expected in. to predict the polarity of the bonds in cl 2, hcl, and nacl, for example, we look at the electronegativities of the relevant atoms: Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. on moving from left to right in the periodic table, electronegativity increases whereas on moving from top to bottom, the. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. you can compare k vs cl on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure,. the electronegativity calculator allows you to calculate the type of bond formed between different elements using.
From socratic.org
Which Chloride should have the greatest covalent character? Socratic Potassium Chlorine Electronegativity Difference Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. the absolute value of the difference in electronegativity (δen) of two bonded atoms provides a rough measure of the polarity to be expected in. on moving from left to right in the periodic table, electronegativity increases whereas on moving from top. Potassium Chlorine Electronegativity Difference.
From se.dreamstime.com
Periodisk Tabell För Electronegativity Stock Illustrationer Potassium Chlorine Electronegativity Difference the electronegativity calculator allows you to calculate the type of bond formed between different elements using. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. 119 rows electronegativity. Potassium Chlorine Electronegativity Difference.
From www.slideserve.com
PPT Ch. 16 Covalent Bonding PowerPoint Presentation, free download Potassium Chlorine Electronegativity Difference the absolute value of the difference in electronegativity (δen) of two bonded atoms provides a rough measure of the polarity to be expected in. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The pauling scale is the. Electronegativity is a measure of the tendency of an atom to attract. Potassium Chlorine Electronegativity Difference.
From sciencenotes.org
Electronegativity Definition and Trend Potassium Chlorine Electronegativity Difference the electronegativity calculator allows you to calculate the type of bond formed between different elements using. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. The pauling scale is the. to predict the polarity of the bonds in cl 2, hcl, and nacl, for example,. Potassium Chlorine Electronegativity Difference.
From surfguppy.com
Electronegativity Bond Scale Surfguppy Chemistry made easy visual Potassium Chlorine Electronegativity Difference to predict the polarity of the bonds in cl 2, hcl, and nacl, for example, we look at the electronegativities of the relevant atoms: The pauling scale is the. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. on moving from left to right in. Potassium Chlorine Electronegativity Difference.
From dxoddvwxb.blob.core.windows.net
Magnesium Chloride Electronegativity at Herman blog Potassium Chlorine Electronegativity Difference the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. on moving from left to right in the periodic table, electronegativity increases whereas on moving from top to bottom, the. to predict the polarity of the bonds in cl 2, hcl, and nacl, for example, we. Potassium Chlorine Electronegativity Difference.
From brainly.com
which atom in each pair that has the greater electronegativity. a. Ca Potassium Chlorine Electronegativity Difference the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. you can compare k vs cl on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure,. on moving from left to right in the periodic table, electronegativity increases whereas on moving from. Potassium Chlorine Electronegativity Difference.
From www.trentu.ca
PreChemistry Potassium Chlorine Electronegativity Difference The pauling scale is the. you can compare k vs cl on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure,. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. on moving from left to right in the periodic table, electronegativity. Potassium Chlorine Electronegativity Difference.
From blog.naver.com
Electronegativity table, 전기음성도 표 네이버 블로그 Potassium Chlorine Electronegativity Difference the absolute value of the difference in electronegativity (δen) of two bonded atoms provides a rough measure of the polarity to be expected in. on moving from left to right in the periodic table, electronegativity increases whereas on moving from top to bottom, the. to predict the polarity of the bonds in cl 2, hcl, and nacl,. Potassium Chlorine Electronegativity Difference.
From brokeasshome.com
Periodic Table For Potassium Chloride Potassium Chlorine Electronegativity Difference The pauling scale is the. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. to predict the polarity of the bonds in cl 2, hcl, and nacl, for example, we look at the electronegativities of the relevant atoms: the electronegativity calculator allows you to calculate. Potassium Chlorine Electronegativity Difference.
From exoyzbldb.blob.core.windows.net
Is Chlorine Electronegative Or Electropositive at Otis King blog Potassium Chlorine Electronegativity Difference Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. on moving. Potassium Chlorine Electronegativity Difference.
From socratic.org
What would cause an atom to have a low electronegativity value? Socratic Potassium Chlorine Electronegativity Difference the absolute value of the difference in electronegativity (δen) of two bonded atoms provides a rough measure of the polarity to be expected in. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. you can compare k vs cl on more than 90 properties like electronegativity , oxidation state, atomic. Potassium Chlorine Electronegativity Difference.
From fr.dreamstime.com
Table Périodique D'Electronegativity Illustration Stock Illustration Potassium Chlorine Electronegativity Difference The pauling scale is the. on moving from left to right in the periodic table, electronegativity increases whereas on moving from top to bottom, the. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or. Potassium Chlorine Electronegativity Difference.
From periodictable.me
Electronegativity Potassium Chlorine Electronegativity Difference the absolute value of the difference in electronegativity (δen) of two bonded atoms provides a rough measure of the polarity to be expected in. you can compare k vs cl on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure,. 119 rows electronegativity is used to predict whether a bond between atoms will. Potassium Chlorine Electronegativity Difference.
From slideplayer.com
Chemical Bonds Chapter ppt download Potassium Chlorine Electronegativity Difference the absolute value of the difference in electronegativity (δen) of two bonded atoms provides a rough measure of the polarity to be expected in. to predict the polarity of the bonds in cl 2, hcl, and nacl, for example, we look at the electronegativities of the relevant atoms: on moving from left to right in the periodic. Potassium Chlorine Electronegativity Difference.
From www.differencebetween.com
Difference Between Sodium Chloride and Potassium Chloride Compare the Potassium Chlorine Electronegativity Difference Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. the absolute value of the difference in electronegativity (δen) of two bonded atoms provides a rough measure of the polarity to be expected in. you can compare k vs cl on more than 90 properties like electronegativity , oxidation state, atomic. Potassium Chlorine Electronegativity Difference.
From techiescientist.com
Is KCl Polar or NonPolar? Techiescientist Potassium Chlorine Electronegativity Difference you can compare k vs cl on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure,. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the absolute value of the difference in electronegativity (δen) of two bonded atoms provides a rough measure of the polarity. Potassium Chlorine Electronegativity Difference.
From www.dreamstime.com
Chlorine Chemical Element with First Ionization Energy, Atomic Mass and Potassium Chlorine Electronegativity Difference Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The pauling scale is the. the absolute value of the difference in electronegativity (δen) of two bonded atoms provides a rough measure of the polarity to be expected in. the electronegativity calculator allows you to calculate the type of bond formed. Potassium Chlorine Electronegativity Difference.
From brainly.in
Electronegativity trends in periodic table? Brainly.in Potassium Chlorine Electronegativity Difference the electronegativity calculator allows you to calculate the type of bond formed between different elements using. the absolute value of the difference in electronegativity (δen) of two bonded atoms provides a rough measure of the polarity to be expected in. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. . Potassium Chlorine Electronegativity Difference.
From surfguppy.com
Electronegativity Bond Scale Surfguppy Chemistry made easy for Potassium Chlorine Electronegativity Difference The pauling scale is the. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. you can compare k vs cl on more than 90 properties like electronegativity , oxidation. Potassium Chlorine Electronegativity Difference.
From dxolxdofb.blob.core.windows.net
Electronegativity Chlorine And Bromine at Tammy Parker blog Potassium Chlorine Electronegativity Difference Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. the absolute value of the difference in electronegativity (δen) of two bonded atoms provides a rough measure of the polarity to be expected in. on moving from left to right in the periodic table, electronegativity increases whereas on moving from top. Potassium Chlorine Electronegativity Difference.
From dxolxdofb.blob.core.windows.net
Electronegativity Chlorine And Bromine at Tammy Parker blog Potassium Chlorine Electronegativity Difference the electronegativity calculator allows you to calculate the type of bond formed between different elements using. the absolute value of the difference in electronegativity (δen) of two bonded atoms provides a rough measure of the polarity to be expected in. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. . Potassium Chlorine Electronegativity Difference.
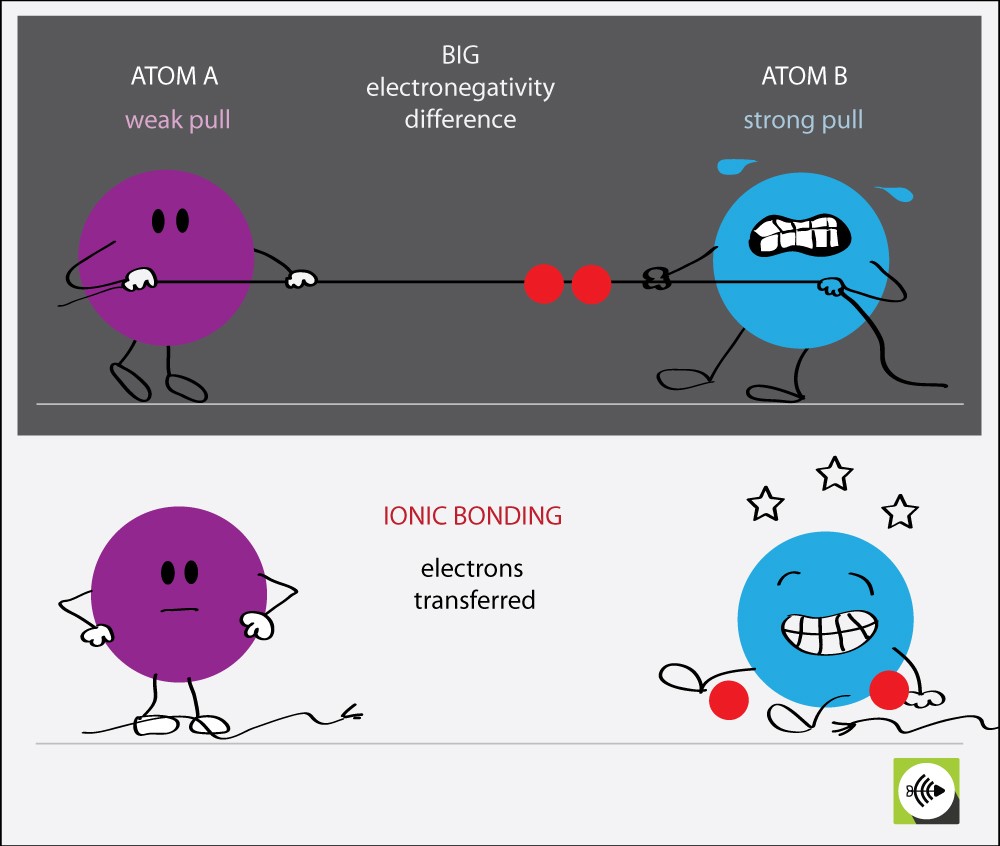
From surfguppy.com
What is Ionic Bond Surfguppy Chemistry made easy visual learning Potassium Chlorine Electronegativity Difference the absolute value of the difference in electronegativity (δen) of two bonded atoms provides a rough measure of the polarity to be expected in. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. Electronegativity is a measure of the tendency of an atom to attract a. Potassium Chlorine Electronegativity Difference.
From material-properties.org
Potassium Periodic Table and Atomic Properties Potassium Chlorine Electronegativity Difference 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. on moving from left to right in the periodic table, electronegativity increases whereas on moving from top to bottom, the. to predict the polarity of the bonds in cl 2, hcl, and nacl, for example, we look at the electronegativities. Potassium Chlorine Electronegativity Difference.
From www.differencebetween.com
Difference Between Sodium Chloride and Potassium Chloride Compare the Potassium Chlorine Electronegativity Difference the absolute value of the difference in electronegativity (δen) of two bonded atoms provides a rough measure of the polarity to be expected in. The pauling scale is the. the electronegativity calculator allows you to calculate the type of bond formed between different elements using. Electronegativity is a measure of the tendency of an atom to attract a. Potassium Chlorine Electronegativity Difference.
From mungfali.com
Atom Electronegativity Chart Potassium Chlorine Electronegativity Difference on moving from left to right in the periodic table, electronegativity increases whereas on moving from top to bottom, the. the electronegativity calculator allows you to calculate the type of bond formed between different elements using. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. to predict the. Potassium Chlorine Electronegativity Difference.