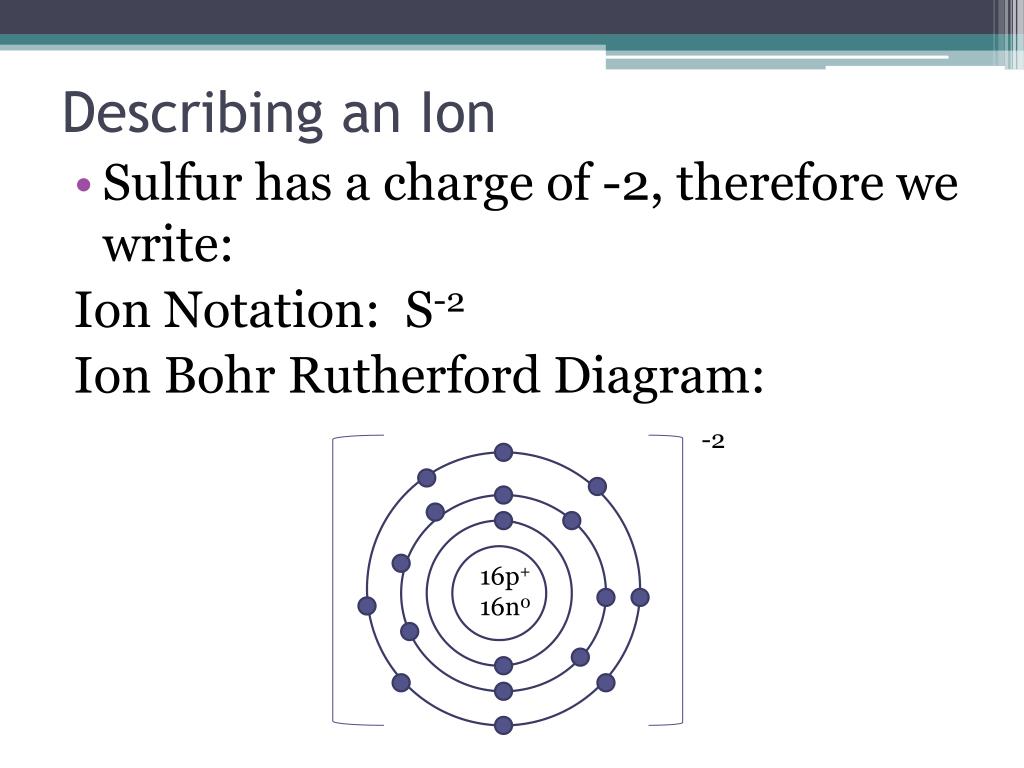
Will S Form A Negative Ion . a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. The ions formed are negative,. an ion with a positive charge is called a cation, while an ion with a negative charge is called an anion. An easy mnemonic to distinguish the two is that the the word. A nitrogen atom must gain three electrons to have the same number of electrons as an. when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. In this ocr gcse chemistry study guide, we'll go through the group 0. in nature, however, many atoms lose or gain electrons and change from being neutral to having a net positive or negative charge. nonmetals form negative ions (anions). Thus, if you commit the. This page shows how some atoms can bond by transferring.
from www.slideserve.com
a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. an ion with a positive charge is called a cation, while an ion with a negative charge is called an anion. in nature, however, many atoms lose or gain electrons and change from being neutral to having a net positive or negative charge. An easy mnemonic to distinguish the two is that the the word. In this ocr gcse chemistry study guide, we'll go through the group 0. when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. Thus, if you commit the. The ions formed are negative,. This page shows how some atoms can bond by transferring. nonmetals form negative ions (anions).
PPT Forming Ions PowerPoint Presentation, free download ID2731910
Will S Form A Negative Ion nonmetals form negative ions (anions). The ions formed are negative,. A nitrogen atom must gain three electrons to have the same number of electrons as an. An easy mnemonic to distinguish the two is that the the word. In this ocr gcse chemistry study guide, we'll go through the group 0. an ion with a positive charge is called a cation, while an ion with a negative charge is called an anion. in nature, however, many atoms lose or gain electrons and change from being neutral to having a net positive or negative charge. This page shows how some atoms can bond by transferring. nonmetals form negative ions (anions). when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Thus, if you commit the.
From brainly.in
how are ions formed explain pls Brainly.in Will S Form A Negative Ion The ions formed are negative,. An easy mnemonic to distinguish the two is that the the word. nonmetals form negative ions (anions). Thus, if you commit the. This page shows how some atoms can bond by transferring. A nitrogen atom must gain three electrons to have the same number of electrons as an. in nature, however, many atoms. Will S Form A Negative Ion.
From utedzz.blogspot.com
Printable Periodic Table Ionic Charges Periodic Table Timeline Will S Form A Negative Ion nonmetals form negative ions (anions). when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. A nitrogen atom must gain three electrons to have the same number of electrons as an. This page shows how some atoms can bond by transferring. an ion with a positive charge. Will S Form A Negative Ion.
From www.myxxgirl.com
Positive And Negative Ions Chart My XXX Hot Girl Will S Form A Negative Ion In this ocr gcse chemistry study guide, we'll go through the group 0. An easy mnemonic to distinguish the two is that the the word. A nitrogen atom must gain three electrons to have the same number of electrons as an. an ion with a positive charge is called a cation, while an ion with a negative charge is. Will S Form A Negative Ion.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Will S Form A Negative Ion in nature, however, many atoms lose or gain electrons and change from being neutral to having a net positive or negative charge. nonmetals form negative ions (anions). an ion with a positive charge is called a cation, while an ion with a negative charge is called an anion. when sodium atoms form ions, they always form. Will S Form A Negative Ion.
From lessonlistfraughtage.z13.web.core.windows.net
How Do Positive And Negative Ions Form Will S Form A Negative Ion when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. This page shows how some atoms can bond by transferring. an ion with a positive charge is called a cation, while an ion with a negative charge is called an anion. in nature, however, many atoms lose. Will S Form A Negative Ion.
From www.slideserve.com
PPT Crystal Binding (Bonding) Continued More on Ionic Bonding Will S Form A Negative Ion This page shows how some atoms can bond by transferring. The ions formed are negative,. A nitrogen atom must gain three electrons to have the same number of electrons as an. when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. nonmetals form negative ions (anions). An easy. Will S Form A Negative Ion.
From tutorbin.com
Solved Complete the table with the positive ion, negative ion, and the Will S Form A Negative Ion The ions formed are negative,. An easy mnemonic to distinguish the two is that the the word. nonmetals form negative ions (anions). in nature, however, many atoms lose or gain electrons and change from being neutral to having a net positive or negative charge. a cation (a positive ion) forms when a neutral atom loses one or. Will S Form A Negative Ion.
From www.nagwa.com
Lesson Video Ions Nagwa Will S Form A Negative Ion The ions formed are negative,. A nitrogen atom must gain three electrons to have the same number of electrons as an. An easy mnemonic to distinguish the two is that the the word. Thus, if you commit the. in nature, however, many atoms lose or gain electrons and change from being neutral to having a net positive or negative. Will S Form A Negative Ion.
From slideplayer.com
1. ppt download Will S Form A Negative Ion nonmetals form negative ions (anions). Thus, if you commit the. in nature, however, many atoms lose or gain electrons and change from being neutral to having a net positive or negative charge. In this ocr gcse chemistry study guide, we'll go through the group 0. when sodium atoms form ions, they always form a 1+ charge, never. Will S Form A Negative Ion.
From socratic.org
Question 6b48e + Example Will S Form A Negative Ion a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. A nitrogen atom must gain three electrons to have the same number of electrons. Will S Form A Negative Ion.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Will S Form A Negative Ion when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. Thus, if you commit the. A nitrogen atom must gain three electrons to have the same number of electrons as an. This page shows how some atoms can bond by transferring. a cation (a positive ion) forms when. Will S Form A Negative Ion.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Will S Form A Negative Ion A nitrogen atom must gain three electrons to have the same number of electrons as an. This page shows how some atoms can bond by transferring. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. when sodium atoms form ions, they always form. Will S Form A Negative Ion.
From www.flinnsci.ca
Ion Names, Formulas and Charges Chart Flinn Scientific Will S Form A Negative Ion This page shows how some atoms can bond by transferring. when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. In this ocr gcse chemistry study guide, we'll go through the group 0. Thus, if you commit the. in nature, however, many atoms lose or gain electrons and. Will S Form A Negative Ion.
From www.purlifeus.com
Purlife Negative Ion Jewelry Accessories Wristbands Will S Form A Negative Ion a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. An easy mnemonic to distinguish the two is that the the word. nonmetals form negative ions (anions). The ions formed are negative,. in nature, however, many atoms lose or gain electrons and change. Will S Form A Negative Ion.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Will S Form A Negative Ion a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. an ion with a positive charge is called a cation, while an ion with a negative charge is called an anion. A nitrogen atom must gain three electrons to have the same number of. Will S Form A Negative Ion.
From healingthroughnature.sg
Negative Ions Science Behind It Healing with Nature Will S Form A Negative Ion This page shows how some atoms can bond by transferring. when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. in nature, however, many atoms lose or gain electrons and change from being neutral to having a net positive or negative charge. nonmetals form negative ions (anions).. Will S Form A Negative Ion.
From www.slideserve.com
PPT To View the presentation as a slideshow with effects PowerPoint Will S Form A Negative Ion a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. in nature, however, many atoms lose or gain electrons and change from being neutral to having a net positive or negative charge. This page shows how some atoms can bond by transferring. An easy. Will S Form A Negative Ion.
From www.slideserve.com
PPT Forming Ions PowerPoint Presentation, free download ID2731910 Will S Form A Negative Ion in nature, however, many atoms lose or gain electrons and change from being neutral to having a net positive or negative charge. An easy mnemonic to distinguish the two is that the the word. A nitrogen atom must gain three electrons to have the same number of electrons as an. nonmetals form negative ions (anions). This page shows. Will S Form A Negative Ion.
From atmemi.pics
Molecular and ionic compounds Chemistry for specialties (2022) Will S Form A Negative Ion A nitrogen atom must gain three electrons to have the same number of electrons as an. An easy mnemonic to distinguish the two is that the the word. an ion with a positive charge is called a cation, while an ion with a negative charge is called an anion. This page shows how some atoms can bond by transferring.. Will S Form A Negative Ion.
From learningschoollavad0s.z14.web.core.windows.net
Positive Ions And Negative Ions Table Will S Form A Negative Ion a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. An easy mnemonic to distinguish the two is that the the word. when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. A nitrogen. Will S Form A Negative Ion.
From www.numerade.com
SOLVEDNonmetals form negative ions by (losing/gaining) enough Will S Form A Negative Ion nonmetals form negative ions (anions). The ions formed are negative,. This page shows how some atoms can bond by transferring. An easy mnemonic to distinguish the two is that the the word. in nature, however, many atoms lose or gain electrons and change from being neutral to having a net positive or negative charge. when sodium atoms. Will S Form A Negative Ion.
From www.differencebetween.com
Difference Between Positive and Negative Ion Compare the Difference Will S Form A Negative Ion In this ocr gcse chemistry study guide, we'll go through the group 0. when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. This page shows how some atoms can bond by transferring. an ion with a positive charge is called a cation, while an ion with a. Will S Form A Negative Ion.
From www.thesciencehive.co.uk
Ionic Bonding — the science sauce Will S Form A Negative Ion A nitrogen atom must gain three electrons to have the same number of electrons as an. when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. The ions formed are negative,. an ion with a positive charge is called a cation, while an ion with a negative charge. Will S Form A Negative Ion.
From www.goodscience.com.au
Formation of Ions and Ionic Compounds Good Science Will S Form A Negative Ion An easy mnemonic to distinguish the two is that the the word. Thus, if you commit the. when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. in nature, however, many atoms lose or gain electrons and change from being neutral to having a net positive or negative. Will S Form A Negative Ion.
From selftution.com
Difference between Ions and Radicals with Examples » Selftution Will S Form A Negative Ion nonmetals form negative ions (anions). Thus, if you commit the. when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. an ion with a positive charge is called a cation, while an ion with a negative charge is called an anion. The ions formed are negative,. This. Will S Form A Negative Ion.
From www.compoundchem.com
Common Polyatomic Ions Names, Formulae, and Charges Compound Interest Will S Form A Negative Ion In this ocr gcse chemistry study guide, we'll go through the group 0. in nature, however, many atoms lose or gain electrons and change from being neutral to having a net positive or negative charge. The ions formed are negative,. when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even. Will S Form A Negative Ion.
From schematicdiagramyakuza.z13.web.core.windows.net
Lewis Dot Symbols For All Elements Will S Form A Negative Ion nonmetals form negative ions (anions). In this ocr gcse chemistry study guide, we'll go through the group 0. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. in nature, however, many atoms lose or gain electrons and change from being neutral to. Will S Form A Negative Ion.
From www.pinterest.com
Ion Names, Formulas and Charges Chart Teaching chemistry, Chemistry Will S Form A Negative Ion The ions formed are negative,. nonmetals form negative ions (anions). Thus, if you commit the. when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion. Will S Form A Negative Ion.
From elanra.com
All about positive and negative ions? Will S Form A Negative Ion a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. The ions formed are negative,. An easy mnemonic to distinguish the two is that the the word. In this ocr gcse chemistry study guide, we'll go through the group 0. This page shows how some. Will S Form A Negative Ion.
From spmchemistry.blog.onlinetuition.com.my
Formation of Ion SPM Chemistry Will S Form A Negative Ion Thus, if you commit the. in nature, however, many atoms lose or gain electrons and change from being neutral to having a net positive or negative charge. A nitrogen atom must gain three electrons to have the same number of electrons as an. a cation (a positive ion) forms when a neutral atom loses one or more electrons. Will S Form A Negative Ion.
From itc.gsw.edu
Ions Will S Form A Negative Ion in nature, however, many atoms lose or gain electrons and change from being neutral to having a net positive or negative charge. A nitrogen atom must gain three electrons to have the same number of electrons as an. In this ocr gcse chemistry study guide, we'll go through the group 0. an ion with a positive charge is. Will S Form A Negative Ion.
From guidediagramterrain.z5.web.core.windows.net
The Lewis Dot Diagram For Ionic Bonding Will S Form A Negative Ion A nitrogen atom must gain three electrons to have the same number of electrons as an. nonmetals form negative ions (anions). in nature, however, many atoms lose or gain electrons and change from being neutral to having a net positive or negative charge. An easy mnemonic to distinguish the two is that the the word. This page shows. Will S Form A Negative Ion.
From www.dochub.com
Ion formation worksheet Fill out & sign online DocHub Will S Form A Negative Ion nonmetals form negative ions (anions). A nitrogen atom must gain three electrons to have the same number of electrons as an. when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. In this ocr gcse chemistry study guide, we'll go through the group 0. The ions formed are. Will S Form A Negative Ion.
From thechemistrynotes.com
Ionic Lattice and Ionic Compounds Will S Form A Negative Ion An easy mnemonic to distinguish the two is that the the word. Thus, if you commit the. The ions formed are negative,. when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. A nitrogen atom must gain three electrons to have the same number of electrons as an. . Will S Form A Negative Ion.
From alevelbiology.co.uk
Ions Types, Summary, Classification & Facts Will S Form A Negative Ion nonmetals form negative ions (anions). Thus, if you commit the. an ion with a positive charge is called a cation, while an ion with a negative charge is called an anion. An easy mnemonic to distinguish the two is that the the word. In this ocr gcse chemistry study guide, we'll go through the group 0. The ions. Will S Form A Negative Ion.