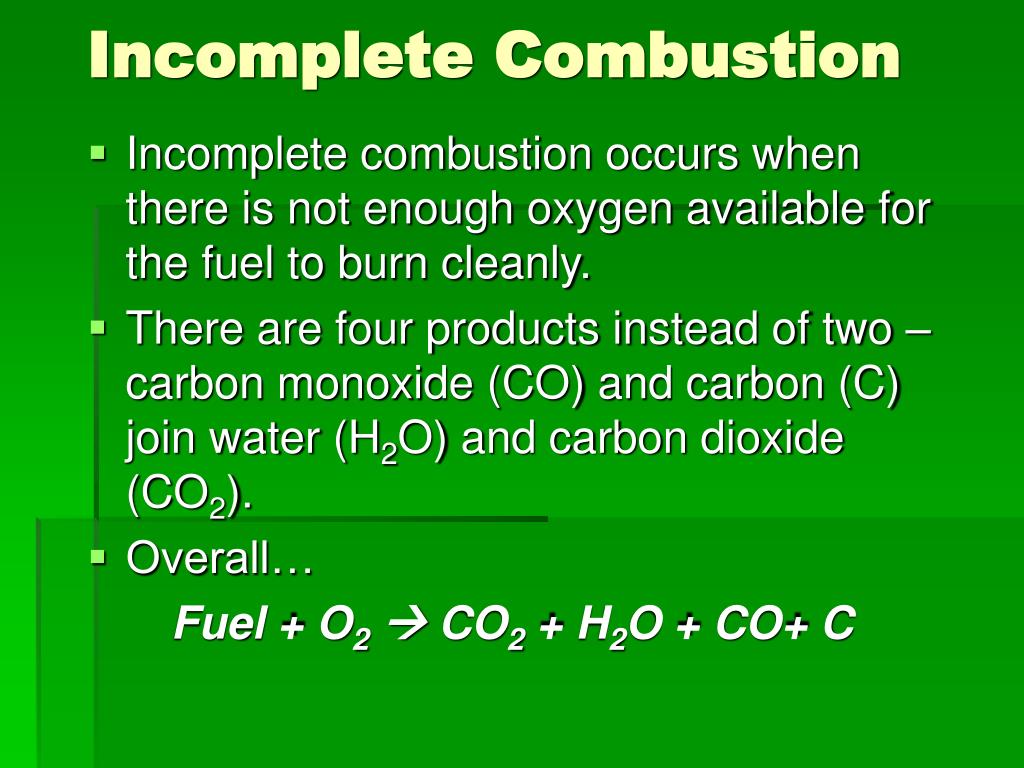
Explain Why Incomplete Combustion Of Methane Is Dangerous . Incomplete combustion occurs when there is insufficient oxygen to burn; When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and not all the carbon is fully oxidised; To construct a symbol equation for the incomplete combustion of a fuel, you need to read the question to see whether carbon monoxide (co). Incomplete combustion (where there is not enough oxygen present) can lead to the formation of carbon or carbon monoxide. It occurs in some appliances such as boilers and stoves as well. Incomplete combustion occurs when there isn’t enough oxygen to allow the fuel to react completely with the oxygen to produce carbon. Incomplete combustion of alkanes in enclosed spaces is a significant safety hazard primarily due to the production of carbon monoxide (co). Complete combustion results in carbon dioxide and water, while incomplete combustion can produce carbon monoxide (co) and other.
from www.slideserve.com
To construct a symbol equation for the incomplete combustion of a fuel, you need to read the question to see whether carbon monoxide (co). Incomplete combustion occurs when there isn’t enough oxygen to allow the fuel to react completely with the oxygen to produce carbon. Incomplete combustion (where there is not enough oxygen present) can lead to the formation of carbon or carbon monoxide. Complete combustion results in carbon dioxide and water, while incomplete combustion can produce carbon monoxide (co) and other. Incomplete combustion occurs when there is insufficient oxygen to burn; It occurs in some appliances such as boilers and stoves as well. When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and not all the carbon is fully oxidised; Incomplete combustion of alkanes in enclosed spaces is a significant safety hazard primarily due to the production of carbon monoxide (co).
PPT Types of Chemical Reactions PowerPoint Presentation, free
Explain Why Incomplete Combustion Of Methane Is Dangerous Incomplete combustion (where there is not enough oxygen present) can lead to the formation of carbon or carbon monoxide. Complete combustion results in carbon dioxide and water, while incomplete combustion can produce carbon monoxide (co) and other. Incomplete combustion of alkanes in enclosed spaces is a significant safety hazard primarily due to the production of carbon monoxide (co). When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and not all the carbon is fully oxidised; It occurs in some appliances such as boilers and stoves as well. To construct a symbol equation for the incomplete combustion of a fuel, you need to read the question to see whether carbon monoxide (co). Incomplete combustion occurs when there is insufficient oxygen to burn; Incomplete combustion (where there is not enough oxygen present) can lead to the formation of carbon or carbon monoxide. Incomplete combustion occurs when there isn’t enough oxygen to allow the fuel to react completely with the oxygen to produce carbon.
From www.slideserve.com
PPT Chapter 9 Energy, Enthalpy and Thermochemistry PowerPoint Explain Why Incomplete Combustion Of Methane Is Dangerous It occurs in some appliances such as boilers and stoves as well. To construct a symbol equation for the incomplete combustion of a fuel, you need to read the question to see whether carbon monoxide (co). Incomplete combustion occurs when there is insufficient oxygen to burn; When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.slideserve.com
PPT ENTHALPY OF FORMATION Combustion of Methanol PowerPoint Explain Why Incomplete Combustion Of Methane Is Dangerous Incomplete combustion occurs when there isn’t enough oxygen to allow the fuel to react completely with the oxygen to produce carbon. It occurs in some appliances such as boilers and stoves as well. When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and not all the carbon is fully oxidised; Complete combustion results. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.youtube.com
Dangers of Combustion GCSE science, Chemistry (91) YouTube Explain Why Incomplete Combustion Of Methane Is Dangerous Incomplete combustion occurs when there is insufficient oxygen to burn; Complete combustion results in carbon dioxide and water, while incomplete combustion can produce carbon monoxide (co) and other. When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and not all the carbon is fully oxidised; To construct a symbol equation for the incomplete. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.tessshebaylo.com
Methane Combustion Word Equation Tessshebaylo Explain Why Incomplete Combustion Of Methane Is Dangerous When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and not all the carbon is fully oxidised; Incomplete combustion of alkanes in enclosed spaces is a significant safety hazard primarily due to the production of carbon monoxide (co). To construct a symbol equation for the incomplete combustion of a fuel, you need to. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.youtube.com
How to Balance CH4 + O2 = CO + C + H2O Combustion of Explain Why Incomplete Combustion Of Methane Is Dangerous Incomplete combustion occurs when there isn’t enough oxygen to allow the fuel to react completely with the oxygen to produce carbon. To construct a symbol equation for the incomplete combustion of a fuel, you need to read the question to see whether carbon monoxide (co). Complete combustion results in carbon dioxide and water, while incomplete combustion can produce carbon monoxide. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From brainly.com
Which of the following reactions show the balanced reaction for the Explain Why Incomplete Combustion Of Methane Is Dangerous Incomplete combustion occurs when there isn’t enough oxygen to allow the fuel to react completely with the oxygen to produce carbon. It occurs in some appliances such as boilers and stoves as well. Complete combustion results in carbon dioxide and water, while incomplete combustion can produce carbon monoxide (co) and other. To construct a symbol equation for the incomplete combustion. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.tessshebaylo.com
Combustion Of Methane Chemical Equation Tessshebaylo Explain Why Incomplete Combustion Of Methane Is Dangerous Incomplete combustion occurs when there is insufficient oxygen to burn; Incomplete combustion occurs when there isn’t enough oxygen to allow the fuel to react completely with the oxygen to produce carbon. It occurs in some appliances such as boilers and stoves as well. When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.tessshebaylo.com
Methane Combustion Word Equation Tessshebaylo Explain Why Incomplete Combustion Of Methane Is Dangerous Incomplete combustion of alkanes in enclosed spaces is a significant safety hazard primarily due to the production of carbon monoxide (co). Incomplete combustion (where there is not enough oxygen present) can lead to the formation of carbon or carbon monoxide. Incomplete combustion occurs when there isn’t enough oxygen to allow the fuel to react completely with the oxygen to produce. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.tessshebaylo.com
What Is The Balanced Symbol Equation For Combustion Of Explain Why Incomplete Combustion Of Methane Is Dangerous Incomplete combustion occurs when there isn’t enough oxygen to allow the fuel to react completely with the oxygen to produce carbon. Complete combustion results in carbon dioxide and water, while incomplete combustion can produce carbon monoxide (co) and other. To construct a symbol equation for the incomplete combustion of a fuel, you need to read the question to see whether. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.youtube.com
Difference between Complete Combustion Vs Combustion Explain Why Incomplete Combustion Of Methane Is Dangerous Incomplete combustion (where there is not enough oxygen present) can lead to the formation of carbon or carbon monoxide. To construct a symbol equation for the incomplete combustion of a fuel, you need to read the question to see whether carbon monoxide (co). When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From methanegaskimawashi.blogspot.com
Methane Gas The Combustion Of Methane Gas Explain Why Incomplete Combustion Of Methane Is Dangerous It occurs in some appliances such as boilers and stoves as well. To construct a symbol equation for the incomplete combustion of a fuel, you need to read the question to see whether carbon monoxide (co). Incomplete combustion occurs when there isn’t enough oxygen to allow the fuel to react completely with the oxygen to produce carbon. When alkanes are. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.hotelsrate.org
Methane Combustion Equation Diy Projects Explain Why Incomplete Combustion Of Methane Is Dangerous To construct a symbol equation for the incomplete combustion of a fuel, you need to read the question to see whether carbon monoxide (co). It occurs in some appliances such as boilers and stoves as well. When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and not all the carbon is fully oxidised;. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.slideserve.com
PPT Combustion PowerPoint Presentation ID1992427 Explain Why Incomplete Combustion Of Methane Is Dangerous It occurs in some appliances such as boilers and stoves as well. To construct a symbol equation for the incomplete combustion of a fuel, you need to read the question to see whether carbon monoxide (co). When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and not all the carbon is fully oxidised;. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.tessshebaylo.com
Combustion Of Methane Chemical Equation Tessshebaylo Explain Why Incomplete Combustion Of Methane Is Dangerous Incomplete combustion of alkanes in enclosed spaces is a significant safety hazard primarily due to the production of carbon monoxide (co). When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and not all the carbon is fully oxidised; Incomplete combustion occurs when there isn’t enough oxygen to allow the fuel to react completely. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.youtube.com
Combustion & CombustionPhysics YouTube Explain Why Incomplete Combustion Of Methane Is Dangerous To construct a symbol equation for the incomplete combustion of a fuel, you need to read the question to see whether carbon monoxide (co). Complete combustion results in carbon dioxide and water, while incomplete combustion can produce carbon monoxide (co) and other. Incomplete combustion of alkanes in enclosed spaces is a significant safety hazard primarily due to the production of. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.thoughtco.com
An Introduction to Combustion (Burning) Reactions Explain Why Incomplete Combustion Of Methane Is Dangerous Incomplete combustion (where there is not enough oxygen present) can lead to the formation of carbon or carbon monoxide. Incomplete combustion of alkanes in enclosed spaces is a significant safety hazard primarily due to the production of carbon monoxide (co). Incomplete combustion occurs when there is insufficient oxygen to burn; It occurs in some appliances such as boilers and stoves. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.slideserve.com
PPT Chemical Reactions An Introduction Chapter 6 PowerPoint Explain Why Incomplete Combustion Of Methane Is Dangerous When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and not all the carbon is fully oxidised; Incomplete combustion of alkanes in enclosed spaces is a significant safety hazard primarily due to the production of carbon monoxide (co). Incomplete combustion (where there is not enough oxygen present) can lead to the formation of. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From mungfali.com
Combustion Of Methane Balanced Equation Explain Why Incomplete Combustion Of Methane Is Dangerous Incomplete combustion occurs when there isn’t enough oxygen to allow the fuel to react completely with the oxygen to produce carbon. Incomplete combustion of alkanes in enclosed spaces is a significant safety hazard primarily due to the production of carbon monoxide (co). It occurs in some appliances such as boilers and stoves as well. Incomplete combustion (where there is not. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.tessshebaylo.com
What Is The Balanced Equation For Combustion Of Methane Explain Why Incomplete Combustion Of Methane Is Dangerous When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and not all the carbon is fully oxidised; To construct a symbol equation for the incomplete combustion of a fuel, you need to read the question to see whether carbon monoxide (co). Incomplete combustion occurs when there is insufficient oxygen to burn; Incomplete combustion. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.tessshebaylo.com
What Is The Equation For Combustion Of Methane Tessshebaylo Explain Why Incomplete Combustion Of Methane Is Dangerous Incomplete combustion occurs when there isn’t enough oxygen to allow the fuel to react completely with the oxygen to produce carbon. It occurs in some appliances such as boilers and stoves as well. To construct a symbol equation for the incomplete combustion of a fuel, you need to read the question to see whether carbon monoxide (co). Complete combustion results. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.tessshebaylo.com
Combustion Methane Balanced Equation Tessshebaylo Explain Why Incomplete Combustion Of Methane Is Dangerous Incomplete combustion occurs when there is insufficient oxygen to burn; When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and not all the carbon is fully oxidised; To construct a symbol equation for the incomplete combustion of a fuel, you need to read the question to see whether carbon monoxide (co). Complete combustion. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.slideserve.com
PPT Combustion PowerPoint Presentation, free download ID1992427 Explain Why Incomplete Combustion Of Methane Is Dangerous Incomplete combustion (where there is not enough oxygen present) can lead to the formation of carbon or carbon monoxide. To construct a symbol equation for the incomplete combustion of a fuel, you need to read the question to see whether carbon monoxide (co). When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.britannica.com
methane Definition, Properties, Uses, & Facts Britannica Explain Why Incomplete Combustion Of Methane Is Dangerous To construct a symbol equation for the incomplete combustion of a fuel, you need to read the question to see whether carbon monoxide (co). Incomplete combustion occurs when there isn’t enough oxygen to allow the fuel to react completely with the oxygen to produce carbon. Incomplete combustion (where there is not enough oxygen present) can lead to the formation of. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.tessshebaylo.com
Equation Combustion Du Methane Tessshebaylo Explain Why Incomplete Combustion Of Methane Is Dangerous When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and not all the carbon is fully oxidised; Incomplete combustion occurs when there is insufficient oxygen to burn; Complete combustion results in carbon dioxide and water, while incomplete combustion can produce carbon monoxide (co) and other. It occurs in some appliances such as boilers. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.slideserve.com
PPT Combustion PowerPoint Presentation ID1992427 Explain Why Incomplete Combustion Of Methane Is Dangerous When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and not all the carbon is fully oxidised; Incomplete combustion occurs when there is insufficient oxygen to burn; It occurs in some appliances such as boilers and stoves as well. Incomplete combustion (where there is not enough oxygen present) can lead to the formation. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.tessshebaylo.com
Methane Combustion Word Equation Tessshebaylo Explain Why Incomplete Combustion Of Methane Is Dangerous Incomplete combustion occurs when there is insufficient oxygen to burn; Incomplete combustion of alkanes in enclosed spaces is a significant safety hazard primarily due to the production of carbon monoxide (co). Incomplete combustion (where there is not enough oxygen present) can lead to the formation of carbon or carbon monoxide. Incomplete combustion occurs when there isn’t enough oxygen to allow. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.tessshebaylo.com
Equation Bilan De La Combustion Du Methane Tessshebaylo Explain Why Incomplete Combustion Of Methane Is Dangerous Incomplete combustion (where there is not enough oxygen present) can lead to the formation of carbon or carbon monoxide. It occurs in some appliances such as boilers and stoves as well. Complete combustion results in carbon dioxide and water, while incomplete combustion can produce carbon monoxide (co) and other. Incomplete combustion occurs when there is insufficient oxygen to burn; Incomplete. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.tessshebaylo.com
Combustion Methane Balanced Equation Tessshebaylo Explain Why Incomplete Combustion Of Methane Is Dangerous Complete combustion results in carbon dioxide and water, while incomplete combustion can produce carbon monoxide (co) and other. When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and not all the carbon is fully oxidised; Incomplete combustion occurs when there isn’t enough oxygen to allow the fuel to react completely with the oxygen. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From slidetodoc.com
DANGERS OF COMBUSTION COMPARING COMBUSTION Complete graphing Explain Why Incomplete Combustion Of Methane Is Dangerous Incomplete combustion of alkanes in enclosed spaces is a significant safety hazard primarily due to the production of carbon monoxide (co). When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and not all the carbon is fully oxidised; Incomplete combustion occurs when there is insufficient oxygen to burn; Complete combustion results in carbon. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation, free Explain Why Incomplete Combustion Of Methane Is Dangerous When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and not all the carbon is fully oxidised; To construct a symbol equation for the incomplete combustion of a fuel, you need to read the question to see whether carbon monoxide (co). It occurs in some appliances such as boilers and stoves as well.. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From bdteletalk.com
Equation For The Combustion Of Methane Explain Why Incomplete Combustion Of Methane Is Dangerous To construct a symbol equation for the incomplete combustion of a fuel, you need to read the question to see whether carbon monoxide (co). It occurs in some appliances such as boilers and stoves as well. When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and not all the carbon is fully oxidised;. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.slideserve.com
PPT Combustion PowerPoint Presentation, free download ID1992427 Explain Why Incomplete Combustion Of Methane Is Dangerous Incomplete combustion of alkanes in enclosed spaces is a significant safety hazard primarily due to the production of carbon monoxide (co). To construct a symbol equation for the incomplete combustion of a fuel, you need to read the question to see whether carbon monoxide (co). Complete combustion results in carbon dioxide and water, while incomplete combustion can produce carbon monoxide. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.youtube.com
The produce of combustion of methane are CLASS 9 Organic Explain Why Incomplete Combustion Of Methane Is Dangerous Incomplete combustion of alkanes in enclosed spaces is a significant safety hazard primarily due to the production of carbon monoxide (co). To construct a symbol equation for the incomplete combustion of a fuel, you need to read the question to see whether carbon monoxide (co). Incomplete combustion occurs when there is insufficient oxygen to burn; When alkanes are burnt in. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.tes.com
Secondary chemistry teaching resources Chemical reactions TES Explain Why Incomplete Combustion Of Methane Is Dangerous Complete combustion results in carbon dioxide and water, while incomplete combustion can produce carbon monoxide (co) and other. To construct a symbol equation for the incomplete combustion of a fuel, you need to read the question to see whether carbon monoxide (co). It occurs in some appliances such as boilers and stoves as well. Incomplete combustion occurs when there is. Explain Why Incomplete Combustion Of Methane Is Dangerous.
From www.pinterest.co.uk
Understanding Combustion of Methane Explain Why Incomplete Combustion Of Methane Is Dangerous Incomplete combustion (where there is not enough oxygen present) can lead to the formation of carbon or carbon monoxide. Incomplete combustion of alkanes in enclosed spaces is a significant safety hazard primarily due to the production of carbon monoxide (co). When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and not all the. Explain Why Incomplete Combustion Of Methane Is Dangerous.