Copper Hydroxide Reaction . the two ions combine together to form an insoluble salt. But isn't it a solid by. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. — copper hydroxide reacts with sulfuric acid to form copper sulfate and. the reaction of hexaaquacopper (ii) ions with hydroxide ions. — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. heating copper hydroxide produces copper oxide, cuo, a black solid. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions.
from www.chegg.com
— copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. the reaction of hexaaquacopper (ii) ions with hydroxide ions. But isn't it a solid by. — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. the two ions combine together to form an insoluble salt. — copper hydroxide reacts with sulfuric acid to form copper sulfate and. heating copper hydroxide produces copper oxide, cuo, a black solid. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali.
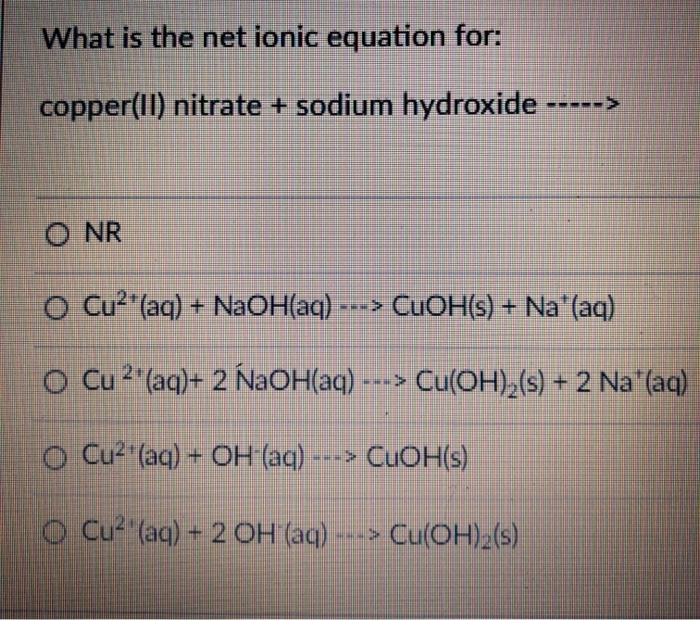
Solved What is the net ionic equation for copper(II)
Copper Hydroxide Reaction — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. heating copper hydroxide produces copper oxide, cuo, a black solid. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. the two ions combine together to form an insoluble salt. — copper hydroxide reacts with sulfuric acid to form copper sulfate and. But isn't it a solid by. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. the reaction of hexaaquacopper (ii) ions with hydroxide ions. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide Reaction Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. — copper hydroxide reacts with sulfuric acid to form copper sulfate and. heating copper hydroxide produces copper oxide, cuo, a black solid. the reaction of hexaaquacopper (ii) ions with hydroxide ions. — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. the production. Copper Hydroxide Reaction.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation Copper Hydroxide Reaction the two ions combine together to form an insoluble salt. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. But isn't it a solid by. the reaction of hexaaquacopper (ii) ions with. Copper Hydroxide Reaction.
From www.youtube.com
Reaction of Sodium Hydroxide and Copper Sulfate YouTube Copper Hydroxide Reaction heating copper hydroxide produces copper oxide, cuo, a black solid. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. — copper hydroxide reacts with sulfuric acid to form copper sulfate and. the two ions combine together to form an insoluble salt. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii). Copper Hydroxide Reaction.
From www.toppr.com
On adding excess of ammonium hydroxide to a copper sulphate solutionA Copper Hydroxide Reaction — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. — copper hydroxide reacts with sulfuric acid to form copper sulfate and. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. the two ions combine together to form an insoluble salt. the production of copper (ii) hydroxide typically occurs through. Copper Hydroxide Reaction.
From www.slideserve.com
PPT Chemical Reactions Copper Reactions PowerPoint Presentation Copper Hydroxide Reaction the two ions combine together to form an insoluble salt. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. heating copper hydroxide. Copper Hydroxide Reaction.
From www.slideserve.com
PPT Thermal Reactions PowerPoint Presentation, free Copper Hydroxide Reaction the two ions combine together to form an insoluble salt. — copper hydroxide reacts with sulfuric acid to form copper sulfate and. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. the reaction of hexaaquacopper (ii) ions with hydroxide ions. Hydroxide ions (from, say, sodium hydroxide solution). Copper Hydroxide Reaction.
From www.youtube.com
Reaction of Copper Sulfate with Ammonium Hydroxide / Ammonia CuSO4 Copper Hydroxide Reaction the two ions combine together to form an insoluble salt. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. heating copper hydroxide produces copper oxide, cuo, a black. Copper Hydroxide Reaction.
From www.doubtnut.com
Copper [II] sulphate solution. Reacts with sodium hydroxide solution to Copper Hydroxide Reaction the two ions combine together to form an insoluble salt. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. But isn't it a solid by. the reaction of hexaaquacopper (ii) ions with hydroxide ions. — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. the production of copper (ii) hydroxide typically occurs through. Copper Hydroxide Reaction.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation Copper Hydroxide Reaction — copper hydroxide reacts with sulfuric acid to form copper sulfate and. — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. the two ions combine together to form an insoluble salt. But isn't it a solid by. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. — copper(ii) ion reacts with stoichiometric. Copper Hydroxide Reaction.
From www.youtube.com
Copper Hydroxide Synthesis YouTube Copper Hydroxide Reaction — copper hydroxide reacts with sulfuric acid to form copper sulfate and. But isn't it a solid by. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. heating copper hydroxide produces copper oxide, cuo, a black solid. the production of. Copper Hydroxide Reaction.
From www.youtube.com
of CopperII Carbonate Hydroxide Hydrate Lab Video YouTube Copper Hydroxide Reaction Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. — copper hydroxide reacts with sulfuric acid to form copper sulfate and. But isn't it a solid by. — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. heating. Copper Hydroxide Reaction.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide Reaction — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. the two ions combine together to form an insoluble salt. — copper hydroxide reacts with sulfuric acid to form. Copper Hydroxide Reaction.
From bnrc.springeropen.com
Copper(II) oxide nanocatalyst preparation and characterization green Copper Hydroxide Reaction But isn't it a solid by. — copper hydroxide reacts with sulfuric acid to form copper sulfate and. the two ions combine together to form an insoluble salt. the reaction of hexaaquacopper (ii) ions with hydroxide ions. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. the production of copper (ii) hydroxide typically occurs through. Copper Hydroxide Reaction.
From www.sciencephoto.com
Copper Hydroxide Precipitate Stock Image C027/9441 Science Photo Copper Hydroxide Reaction — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. But isn't it a solid by. the two ions combine together to form an insoluble salt. heating copper hydroxide produces copper oxide, cuo, a black solid. the production of copper (ii). Copper Hydroxide Reaction.
From brainly.in
Copper sulphate reacts with sodium hydroxide to form blue precipitate Copper Hydroxide Reaction the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. heating copper hydroxide produces copper oxide, cuo, a black solid. — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. the reaction of hexaaquacopper (ii) ions with hydroxide ions. the two ions combine together to. Copper Hydroxide Reaction.
From exorggbgv.blob.core.windows.net
Copper Ii Sulfate And Ammonium Hydroxide Net Ionic Equation at Jennifer Copper Hydroxide Reaction the two ions combine together to form an insoluble salt. — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. — copper hydroxide reacts with sulfuric acid to form copper sulfate and. But isn't it a solid by. — copper(ii) ion reacts with stoichiometric. Copper Hydroxide Reaction.
From fphoto.photoshelter.com
science chemistry precipitation reaction cupric hydroxide Fundamental Copper Hydroxide Reaction the reaction of hexaaquacopper (ii) ions with hydroxide ions. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. But isn't it a solid by. — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii). Copper Hydroxide Reaction.
From stock.adobe.com
Double displacement reaction sodium hydroxide and copper sulfate Copper Hydroxide Reaction — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. — copper hydroxide reacts with sulfuric acid to form copper sulfate and. the reaction of hexaaquacopper (ii) ions with hydroxide ions. heating copper hydroxide produces copper oxide, cuo, a black solid. — copper(ii). Copper Hydroxide Reaction.
From www.mdpi.com
Catalysts Free FullText Layered Copper Hydroxide Salts as Catalyst Copper Hydroxide Reaction — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. the two ions combine together to form an insoluble salt. heating copper hydroxide produces copper oxide,. Copper Hydroxide Reaction.
From www.numerade.com
SOLVED REACTION5COPPER SULFATE(CuSO,)AND SODIUM HYDROXIDE(NaOH) (Write Copper Hydroxide Reaction Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. But isn't it a solid by. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. the two ions combine together to form an. Copper Hydroxide Reaction.
From fineartamerica.com
Copper Hydroxide Precipitate Photograph by Andrew Lambert Photography Copper Hydroxide Reaction heating copper hydroxide produces copper oxide, cuo, a black solid. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. — copper hydroxide reacts with sulfuric acid to form copper sulfate and. the two ions. Copper Hydroxide Reaction.
From www.youtube.com
Making Copper Hydroxide YouTube Copper Hydroxide Reaction the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. the two ions combine together to form an insoluble salt. the reaction of hexaaquacopper (ii) ions with hydroxide ions. But isn't it a solid by. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light. Copper Hydroxide Reaction.
From www.youtube.com
The Reaction Between Copper (II) Nitrate and Sodium Hydroxide YouTube Copper Hydroxide Reaction But isn't it a solid by. — copper hydroxide reacts with sulfuric acid to form copper sulfate and. heating copper hydroxide produces copper oxide, cuo, a black solid. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. . Copper Hydroxide Reaction.
From dxogtzdjb.blob.core.windows.net
Copper Hydroxide Is Soluble In Water at Linwood blog Copper Hydroxide Reaction heating copper hydroxide produces copper oxide, cuo, a black solid. — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. But isn't it a solid by. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. — copper hydroxide reacts with sulfuric acid to form copper. Copper Hydroxide Reaction.
From www.chegg.com
Solved What is the net ionic equation for copper(II) Copper Hydroxide Reaction heating copper hydroxide produces copper oxide, cuo, a black solid. the reaction of hexaaquacopper (ii) ions with hydroxide ions. the two ions combine together to form an insoluble salt. — copper hydroxide reacts with sulfuric acid to form copper sulfate and. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue. Copper Hydroxide Reaction.
From www.chegg.com
Solved 35. Copper(II) hydroxide, For the reaction below, Copper Hydroxide Reaction the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. But isn't it a solid by. the reaction of hexaaquacopper (ii) ions with hydroxide ions. the two ions combine together to form an insoluble salt. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light. Copper Hydroxide Reaction.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation Copper Hydroxide Reaction — copper hydroxide reacts with sulfuric acid to form copper sulfate and. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. But isn't it a solid by. the two ions combine together to form an insoluble salt. — so just. Copper Hydroxide Reaction.
From www.youtube.com
Reaction between copper sulphate(cuso4) and sodium hydroxide(naoh Copper Hydroxide Reaction the two ions combine together to form an insoluble salt. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. heating copper hydroxide produces copper oxide, cuo, a black solid. the reaction of hexaaquacopper (ii) ions with hydroxide ions. — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. — copper(ii) ion reacts. Copper Hydroxide Reaction.
From testbook.com
Copper Hydroxide Learn Definition, Structure, Formula, Uses here Copper Hydroxide Reaction — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. But isn't it a solid by. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. the two ions combine together to form an insoluble salt. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia. Copper Hydroxide Reaction.
From www.youtube.com
Reaction between copper(II)sulfate and potassium hydroxide YouTube Copper Hydroxide Reaction the reaction of hexaaquacopper (ii) ions with hydroxide ions. But isn't it a solid by. the two ions combine together to form an insoluble salt. — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. heating copper hydroxide produces copper oxide, cuo, a black solid. the production of copper (ii) hydroxide typically occurs. Copper Hydroxide Reaction.
From ar.inspiredpencil.com
Copper Hydroxide Solution Copper Hydroxide Reaction the reaction of hexaaquacopper (ii) ions with hydroxide ions. — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. the two ions combine together to form an insoluble salt. — copper hydroxide reacts with sulfuric acid to form copper sulfate and. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. But isn't it. Copper Hydroxide Reaction.
From www.youtube.com
How to Balance Cu(OH)2= CuO + H2O YouTube Copper Hydroxide Reaction the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. — copper hydroxide reacts with sulfuric acid to form copper sulfate and. the reaction of hexaaquacopper (ii) ions with hydroxide ions. But isn't it a solid by. — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's. Copper Hydroxide Reaction.
From www.youtube.com
What kind of reaction is Copper(II) nitrate (Cu(NO3)2) and Sodium Copper Hydroxide Reaction heating copper hydroxide produces copper oxide, cuo, a black solid. — copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. But isn't it a solid by. the two ions combine together to form. Copper Hydroxide Reaction.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide Reaction the two ions combine together to form an insoluble salt. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. the reaction of hexaaquacopper (ii) ions with hydroxide ions. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. — copper(ii) ion reacts with stoichiometric quantities of aqueous. Copper Hydroxide Reaction.
From www.youtube.com
3. Copper Hydroxide to Copper Oxide (Cu Again Lab) YouTube Copper Hydroxide Reaction the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. the two ions combine together to form an insoluble salt. — so just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. the reaction of hexaaquacopper (ii) ions with hydroxide ions. — copper hydroxide reacts with sulfuric. Copper Hydroxide Reaction.