Cu Electroplating Reaction . Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help of an electrolyte bath. An object plated with copper sits in an electrolyte bath with a solution. Electroplating is the process of plating one metal onto another by hydrolysis, most commonly for decorative purposes or to prevent. Includes kit list and safety instructions. Electroplating, also known as electrodeposition, is the process of depositing one metal onto another through controlled electrolysis. This tutorial examines the concept of copper electroplating and how the process works, as well as its use in advanced. Electroplating with copper involves using electrolysis to apply a thin layer of copper onto a conductive surface. Explore the electrolysis of copper (ii) sulfate solution and related industrial processes with this class experiment. In its most basic form, copper electroplating uses electrical current to transfer copper from a copper cathode via electrolysis to the anode, made of another metal. This process results in a thin layer of precious.
from schoolbag.info
Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help of an electrolyte bath. Electroplating is the process of plating one metal onto another by hydrolysis, most commonly for decorative purposes or to prevent. Electroplating, also known as electrodeposition, is the process of depositing one metal onto another through controlled electrolysis. This process results in a thin layer of precious. Explore the electrolysis of copper (ii) sulfate solution and related industrial processes with this class experiment. Electroplating with copper involves using electrolysis to apply a thin layer of copper onto a conductive surface. In its most basic form, copper electroplating uses electrical current to transfer copper from a copper cathode via electrolysis to the anode, made of another metal. An object plated with copper sits in an electrolyte bath with a solution. Includes kit list and safety instructions. This tutorial examines the concept of copper electroplating and how the process works, as well as its use in advanced.
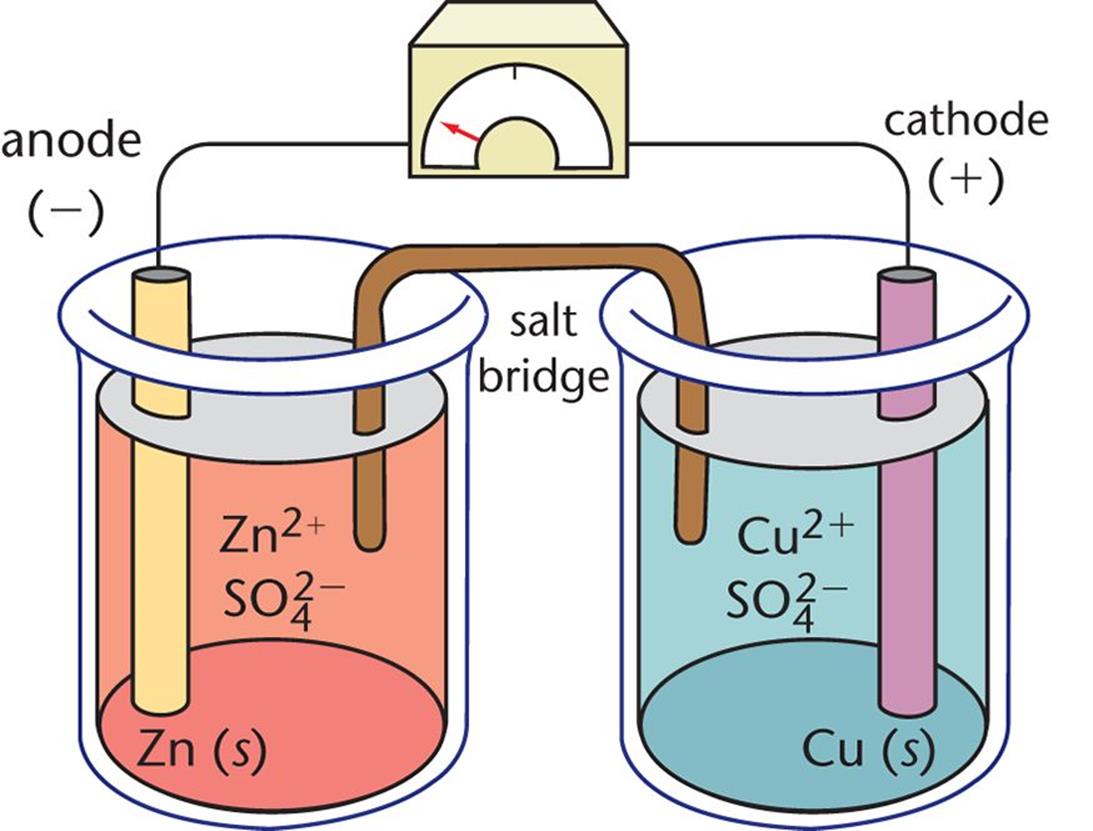
Figure 12.1. Daniell Cell In this galvanic cell, zinc is the anode and
Cu Electroplating Reaction Explore the electrolysis of copper (ii) sulfate solution and related industrial processes with this class experiment. Electroplating, also known as electrodeposition, is the process of depositing one metal onto another through controlled electrolysis. In its most basic form, copper electroplating uses electrical current to transfer copper from a copper cathode via electrolysis to the anode, made of another metal. An object plated with copper sits in an electrolyte bath with a solution. Explore the electrolysis of copper (ii) sulfate solution and related industrial processes with this class experiment. Includes kit list and safety instructions. This process results in a thin layer of precious. This tutorial examines the concept of copper electroplating and how the process works, as well as its use in advanced. Electroplating with copper involves using electrolysis to apply a thin layer of copper onto a conductive surface. Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help of an electrolyte bath. Electroplating is the process of plating one metal onto another by hydrolysis, most commonly for decorative purposes or to prevent.
From www.dreamstime.com
Electroplating with Copper Using Copper Sulfate Electrolyte Stock Cu Electroplating Reaction Explore the electrolysis of copper (ii) sulfate solution and related industrial processes with this class experiment. This tutorial examines the concept of copper electroplating and how the process works, as well as its use in advanced. Electroplating with copper involves using electrolysis to apply a thin layer of copper onto a conductive surface. Electroplating, also known as electrodeposition, is the. Cu Electroplating Reaction.
From spmscience.blog.onlinetuition.com.my
5.5.2 Electrolysis of Copper (II) Chloride Solution SPM Science Cu Electroplating Reaction Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help of an electrolyte bath. Electroplating, also known as electrodeposition, is the process of depositing one metal onto another through controlled electrolysis. Electroplating with copper involves using electrolysis to apply a thin layer of copper onto a conductive surface. This process. Cu Electroplating Reaction.
From mantavya.com
What Is Electroplating & How does it work 2021 Guide Mantavya Cu Electroplating Reaction In its most basic form, copper electroplating uses electrical current to transfer copper from a copper cathode via electrolysis to the anode, made of another metal. Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help of an electrolyte bath. This process results in a thin layer of precious. An. Cu Electroplating Reaction.
From saylordotorg.github.io
Electrochemistry Cu Electroplating Reaction An object plated with copper sits in an electrolyte bath with a solution. Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help of an electrolyte bath. In its most basic form, copper electroplating uses electrical current to transfer copper from a copper cathode via electrolysis to the anode, made. Cu Electroplating Reaction.
From www.nagwa.com
Question Video Identifying the Setup Appropriate for Electroplating Cu Electroplating Reaction Electroplating with copper involves using electrolysis to apply a thin layer of copper onto a conductive surface. In its most basic form, copper electroplating uses electrical current to transfer copper from a copper cathode via electrolysis to the anode, made of another metal. Electroplating is the process of plating one metal onto another by hydrolysis, most commonly for decorative purposes. Cu Electroplating Reaction.
From www.researchgate.net
Electroplating CuZn on Cufoam. a Schematic diagram of Electroplating Cu Electroplating Reaction An object plated with copper sits in an electrolyte bath with a solution. Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help of an electrolyte bath. Electroplating is the process of plating one metal onto another by hydrolysis, most commonly for decorative purposes or to prevent. Electroplating with copper. Cu Electroplating Reaction.
From www.dupont.com
Copper pillar electroplating tutorial Cu Electroplating Reaction Electroplating, also known as electrodeposition, is the process of depositing one metal onto another through controlled electrolysis. In its most basic form, copper electroplating uses electrical current to transfer copper from a copper cathode via electrolysis to the anode, made of another metal. An object plated with copper sits in an electrolyte bath with a solution. Includes kit list and. Cu Electroplating Reaction.
From www.youtube.com
What is Electroplating? Copper Electroplating YouTube Cu Electroplating Reaction This process results in a thin layer of precious. Electroplating, also known as electrodeposition, is the process of depositing one metal onto another through controlled electrolysis. In its most basic form, copper electroplating uses electrical current to transfer copper from a copper cathode via electrolysis to the anode, made of another metal. Electroplating is the process of plating one metal. Cu Electroplating Reaction.
From www.slideserve.com
PPT Electroplating PowerPoint Presentation, free download ID1994006 Cu Electroplating Reaction Includes kit list and safety instructions. Electroplating with copper involves using electrolysis to apply a thin layer of copper onto a conductive surface. This process results in a thin layer of precious. Explore the electrolysis of copper (ii) sulfate solution and related industrial processes with this class experiment. An object plated with copper sits in an electrolyte bath with a. Cu Electroplating Reaction.
From ar.inspiredpencil.com
Electroplating Copper Cu Electroplating Reaction An object plated with copper sits in an electrolyte bath with a solution. Electroplating, also known as electrodeposition, is the process of depositing one metal onto another through controlled electrolysis. This tutorial examines the concept of copper electroplating and how the process works, as well as its use in advanced. Electroplating with copper involves using electrolysis to apply a thin. Cu Electroplating Reaction.
From www.researchgate.net
Schematic representation of copper electroplating setup. Download Cu Electroplating Reaction Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help of an electrolyte bath. An object plated with copper sits in an electrolyte bath with a solution. Electroplating, also known as electrodeposition, is the process of depositing one metal onto another through controlled electrolysis. Includes kit list and safety instructions.. Cu Electroplating Reaction.
From www.researchgate.net
a Schematic diagram of the electroless plating process of CuSnAg Cu Electroplating Reaction An object plated with copper sits in an electrolyte bath with a solution. Explore the electrolysis of copper (ii) sulfate solution and related industrial processes with this class experiment. This tutorial examines the concept of copper electroplating and how the process works, as well as its use in advanced. In its most basic form, copper electroplating uses electrical current to. Cu Electroplating Reaction.
From www.chemistrylearner.com
Electroplating Definition, Process, Example, and Equation Cu Electroplating Reaction This process results in a thin layer of precious. Includes kit list and safety instructions. Electroplating, also known as electrodeposition, is the process of depositing one metal onto another through controlled electrolysis. Electroplating is the process of plating one metal onto another by hydrolysis, most commonly for decorative purposes or to prevent. Copper electroplating is a simple electrochemical process that. Cu Electroplating Reaction.
From www.youtube.com
BASICS OF ELECTROPLATING OF SILVER OVER COPPER AND REDOX REACTIONS Cu Electroplating Reaction Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help of an electrolyte bath. Electroplating is the process of plating one metal onto another by hydrolysis, most commonly for decorative purposes or to prevent. Includes kit list and safety instructions. In its most basic form, copper electroplating uses electrical current. Cu Electroplating Reaction.
From schoolbag.info
Figure 12.1. Daniell Cell In this galvanic cell, zinc is the anode and Cu Electroplating Reaction An object plated with copper sits in an electrolyte bath with a solution. Includes kit list and safety instructions. This tutorial examines the concept of copper electroplating and how the process works, as well as its use in advanced. Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help of. Cu Electroplating Reaction.
From www.slideserve.com
PPT Chapter 20 Principles of Chemical Reactivity Electron Transfer Cu Electroplating Reaction This tutorial examines the concept of copper electroplating and how the process works, as well as its use in advanced. Explore the electrolysis of copper (ii) sulfate solution and related industrial processes with this class experiment. Electroplating is the process of plating one metal onto another by hydrolysis, most commonly for decorative purposes or to prevent. In its most basic. Cu Electroplating Reaction.
From byjus.com
Electrolysis of cuso4 solution using copper as electrode Cu Electroplating Reaction This tutorial examines the concept of copper electroplating and how the process works, as well as its use in advanced. Electroplating, also known as electrodeposition, is the process of depositing one metal onto another through controlled electrolysis. An object plated with copper sits in an electrolyte bath with a solution. Copper electroplating is a simple electrochemical process that results in. Cu Electroplating Reaction.
From 2012books.lardbucket.org
Electrochemistry Cu Electroplating Reaction This tutorial examines the concept of copper electroplating and how the process works, as well as its use in advanced. Explore the electrolysis of copper (ii) sulfate solution and related industrial processes with this class experiment. Electroplating is the process of plating one metal onto another by hydrolysis, most commonly for decorative purposes or to prevent. Includes kit list and. Cu Electroplating Reaction.
From www.youtube.com
Introduction to Electroplating Electrochemistry YouTube Cu Electroplating Reaction This process results in a thin layer of precious. Includes kit list and safety instructions. Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help of an electrolyte bath. Explore the electrolysis of copper (ii) sulfate solution and related industrial processes with this class experiment. An object plated with copper. Cu Electroplating Reaction.
From letstalkscience.ca
Chemical Reactions Conditions & Speeds Let's Talk Science Cu Electroplating Reaction Electroplating, also known as electrodeposition, is the process of depositing one metal onto another through controlled electrolysis. Electroplating with copper involves using electrolysis to apply a thin layer of copper onto a conductive surface. Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help of an electrolyte bath. Electroplating is. Cu Electroplating Reaction.
From www.alamy.com
Electroplating with copper using copper sulfate electrolyte Cu Electroplating Reaction Electroplating, also known as electrodeposition, is the process of depositing one metal onto another through controlled electrolysis. Electroplating is the process of plating one metal onto another by hydrolysis, most commonly for decorative purposes or to prevent. This tutorial examines the concept of copper electroplating and how the process works, as well as its use in advanced. Copper electroplating is. Cu Electroplating Reaction.
From www.slideserve.com
PPT Outline Curriculum (5 lectures) Each lecture 45 minutes Cu Electroplating Reaction This process results in a thin layer of precious. An object plated with copper sits in an electrolyte bath with a solution. In its most basic form, copper electroplating uses electrical current to transfer copper from a copper cathode via electrolysis to the anode, made of another metal. Electroplating, also known as electrodeposition, is the process of depositing one metal. Cu Electroplating Reaction.
From www.youtube.com
Electroplating & The Purification Of Copper (GCSE Chemistry) YouTube Cu Electroplating Reaction This tutorial examines the concept of copper electroplating and how the process works, as well as its use in advanced. Electroplating is the process of plating one metal onto another by hydrolysis, most commonly for decorative purposes or to prevent. Electroplating, also known as electrodeposition, is the process of depositing one metal onto another through controlled electrolysis. This process results. Cu Electroplating Reaction.
From futuros.abrelatam.org
Explain Electrolysis Of Molten Copper Sulphate Using Inert, 47 OFF Cu Electroplating Reaction Electroplating with copper involves using electrolysis to apply a thin layer of copper onto a conductive surface. Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help of an electrolyte bath. Electroplating, also known as electrodeposition, is the process of depositing one metal onto another through controlled electrolysis. This process. Cu Electroplating Reaction.
From www.researchgate.net
The reaction principle of copper electroplating. Download Scientific Cu Electroplating Reaction This tutorial examines the concept of copper electroplating and how the process works, as well as its use in advanced. Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help of an electrolyte bath. Electroplating, also known as electrodeposition, is the process of depositing one metal onto another through controlled. Cu Electroplating Reaction.
From www.youtube.com
Electroplating a key with copper The Real Chemist YouTube Cu Electroplating Reaction Electroplating is the process of plating one metal onto another by hydrolysis, most commonly for decorative purposes or to prevent. Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help of an electrolyte bath. Includes kit list and safety instructions. Electroplating with copper involves using electrolysis to apply a thin. Cu Electroplating Reaction.
From chem2u.blogspot.com
chem2U Electroplating A Coin with Copper Cu Electroplating Reaction Electroplating, also known as electrodeposition, is the process of depositing one metal onto another through controlled electrolysis. An object plated with copper sits in an electrolyte bath with a solution. Electroplating with copper involves using electrolysis to apply a thin layer of copper onto a conductive surface. Copper electroplating is a simple electrochemical process that results in a thin coating. Cu Electroplating Reaction.
From www.mdpi.com
Micromachines Free FullText Study of Copper Electrodeposition at a Cu Electroplating Reaction An object plated with copper sits in an electrolyte bath with a solution. Explore the electrolysis of copper (ii) sulfate solution and related industrial processes with this class experiment. This process results in a thin layer of precious. Electroplating with copper involves using electrolysis to apply a thin layer of copper onto a conductive surface. Electroplating is the process of. Cu Electroplating Reaction.
From 2012books.lardbucket.org
Electrolysis Cu Electroplating Reaction In its most basic form, copper electroplating uses electrical current to transfer copper from a copper cathode via electrolysis to the anode, made of another metal. Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help of an electrolyte bath. An object plated with copper sits in an electrolyte bath. Cu Electroplating Reaction.
From www.researchgate.net
Schematics of shape variation in the electroplated Cu pillar by (a Cu Electroplating Reaction Includes kit list and safety instructions. Electroplating is the process of plating one metal onto another by hydrolysis, most commonly for decorative purposes or to prevent. This tutorial examines the concept of copper electroplating and how the process works, as well as its use in advanced. Electroplating with copper involves using electrolysis to apply a thin layer of copper onto. Cu Electroplating Reaction.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry Cu Electroplating Reaction Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with the help of an electrolyte bath. This process results in a thin layer of precious. This tutorial examines the concept of copper electroplating and how the process works, as well as its use in advanced. Electroplating with copper involves using electrolysis to. Cu Electroplating Reaction.
From wisc.pb.unizin.org
Day 38 OxidationReduction Reactions, Voltaic Cells Chemistry 109 Cu Electroplating Reaction Electroplating with copper involves using electrolysis to apply a thin layer of copper onto a conductive surface. This tutorial examines the concept of copper electroplating and how the process works, as well as its use in advanced. Electroplating, also known as electrodeposition, is the process of depositing one metal onto another through controlled electrolysis. Includes kit list and safety instructions.. Cu Electroplating Reaction.
From www.researchgate.net
Schematic illustration of the principle of sealing the TWH with Cu Cu Electroplating Reaction An object plated with copper sits in an electrolyte bath with a solution. This tutorial examines the concept of copper electroplating and how the process works, as well as its use in advanced. This process results in a thin layer of precious. Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with. Cu Electroplating Reaction.
From www.chemedx.org
An Easy Copper Electroplating Demo for Your Redox Unit Chemical Cu Electroplating Reaction In its most basic form, copper electroplating uses electrical current to transfer copper from a copper cathode via electrolysis to the anode, made of another metal. Includes kit list and safety instructions. This process results in a thin layer of precious. This tutorial examines the concept of copper electroplating and how the process works, as well as its use in. Cu Electroplating Reaction.
From www.slideserve.com
PPT Electroplating PowerPoint Presentation, free download ID2326004 Cu Electroplating Reaction Explore the electrolysis of copper (ii) sulfate solution and related industrial processes with this class experiment. Electroplating, also known as electrodeposition, is the process of depositing one metal onto another through controlled electrolysis. This process results in a thin layer of precious. Copper electroplating is a simple electrochemical process that results in a thin coating on any conductive surface with. Cu Electroplating Reaction.