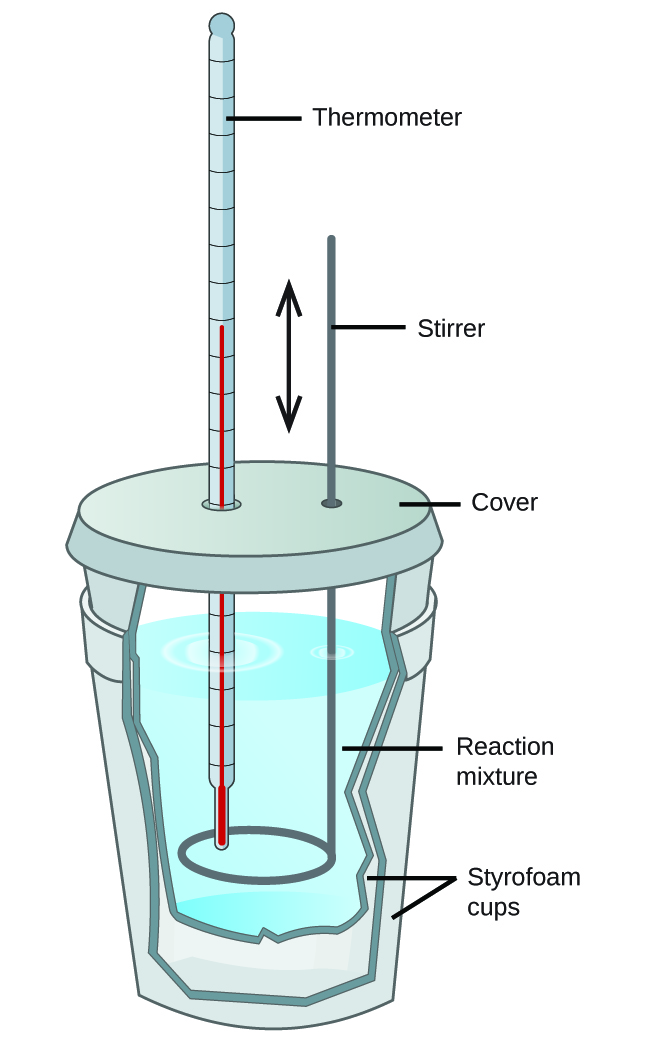
Experiment Calorimetry . Calorimetry is used to measure amounts of heat transferred to or. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Enthalpy changes of reactions in solution. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. There are two types of calorimetry experiments you need to know: 30% of the energy released during the combustion is. To determine the enthalpy, stability, heat capacity (1) what is the purpose of this experiment? Experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. In a calorimetry experiment, 2.50 g of methane is burnt in excess oxygen. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is the science of measuring heat flow. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat.
from
Calorimetry is the science of measuring heat flow. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Enthalpy changes of reactions in solution. There are two types of calorimetry experiments you need to know: 30% of the energy released during the combustion is. Experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a. In a calorimetry experiment, 2.50 g of methane is burnt in excess oxygen. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
Experiment Calorimetry In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a. Calorimetry is the science of measuring heat flow. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Enthalpy changes of reactions in solution. Experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. (1) what is the purpose of this experiment? There are two types of calorimetry experiments you need to know: To determine the enthalpy, stability, heat capacity Calorimetry is used to measure amounts of heat transferred to or. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. 30% of the energy released during the combustion is. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. In a calorimetry experiment, 2.50 g of methane is burnt in excess oxygen. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Experiment Calorimetry Experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. Calorimetry is the science of measuring heat flow. To determine the enthalpy, stability, heat capacity In a calorimetry experiment, 2.50 g of methane is burnt in excess oxygen. (1) what is the purpose of this experiment? One technique we can use to. Experiment Calorimetry.
From
Experiment Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. (1) what is the purpose of this experiment? In a calorimetry experiment, 2.50 g of methane is burnt in excess oxygen. Calorimetry is the science of measuring heat flow. For example, when an exothermic reaction occurs in solution in a calorimeter,. Experiment Calorimetry.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Experiment Calorimetry There are two types of calorimetry experiments you need to know: In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Experiment. Experiment Calorimetry.
From
Experiment Calorimetry In a calorimetry experiment, 2.50 g of methane is burnt in excess oxygen. Experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a. To determine the. Experiment Calorimetry.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Experiment Calorimetry Calorimetry is used to measure amounts of heat transferred to or. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. 30% of the energy released during the combustion is.. Experiment Calorimetry.
From
Experiment Calorimetry One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. (1) what is the purpose of this experiment? In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a. In a calorimetry experiment,. Experiment Calorimetry.
From
Experiment Calorimetry In a calorimetry experiment, 2.50 g of methane is burnt in excess oxygen. Experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. One technique we can use. Experiment Calorimetry.
From
Experiment Calorimetry One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes.. Experiment Calorimetry.
From
Experiment Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is used to measure amounts of heat transferred to or. Calorimetry is the science of measuring. Experiment Calorimetry.
From
Experiment Calorimetry (1) what is the purpose of this experiment? There are two types of calorimetry experiments you need to know: For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. Calorimetry is the science of measuring heat flow. Experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. 30%. Experiment Calorimetry.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Experiment Calorimetry There are two types of calorimetry experiments you need to know: Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. To determine the enthalpy, stability, heat capacity In a calorimetry experiment, 2.50 g of methane is burnt in excess oxygen. Calorimetry is the science of. Experiment Calorimetry.
From
Experiment Calorimetry Enthalpy changes of reactions in solution. Calorimetry is the science of measuring heat flow. In a calorimetry experiment, 2.50 g of methane is burnt in excess oxygen. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand. Experiment Calorimetry.
From
Experiment Calorimetry There are two types of calorimetry experiments you need to know: Experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. To determine the enthalpy, stability, heat capacity Calorimetry is a branch of science concerned with measuring a. Experiment Calorimetry.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Experiment Calorimetry 30% of the energy released during the combustion is. (1) what is the purpose of this experiment? Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. Experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. One technique we can use to. Experiment Calorimetry.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Experiment Calorimetry Calorimetry is the science of measuring heat flow. There are two types of calorimetry experiments you need to know: A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. Experiment Calorimetry.
From studylib.net
experiment 9 Thermochemistry Calorimetry Experiment Calorimetry For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. In a calorimetry experiment, 2.50 g of methane is burnt in excess oxygen. Calorimetry is the science of measuring heat flow. There. Experiment Calorimetry.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Experiment Calorimetry For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. There are two types of calorimetry experiments you need to know: To determine the enthalpy, stability, heat capacity 30% of the energy released during the combustion is. Enthalpy changes of reactions in solution. Calorimetry is used to measure amounts of heat transferred to or. In a. Experiment Calorimetry.
From
Experiment Calorimetry One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is the science of measuring heat flow. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. (1) what is the purpose of this experiment? In this experiment. Experiment Calorimetry.
From
Experiment Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the science of measuring heat flow. 30% of the energy released during the combustion is. Enthalpy changes of reactions in solution. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. (1) what is the. Experiment Calorimetry.
From
Experiment Calorimetry Experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. Enthalpy changes of reactions in solution. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is used to measure amounts of heat transferred to or. 30%. Experiment Calorimetry.
From
Experiment Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. Experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. One technique we can. Experiment Calorimetry.
From
Experiment Calorimetry Experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is used to measure amounts of heat transferred to or. There are two types of calorimetry experiments you need to know: 30%. Experiment Calorimetry.
From
Experiment Calorimetry For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. Experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. (1) what is the purpose of this experiment? 30% of the energy released during the combustion is. A calorimeter is a device used to measure the amount of. Experiment Calorimetry.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Experiment Calorimetry Calorimetry is used to measure amounts of heat transferred to or. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Heat is defined as thermal energy flowing from. Experiment Calorimetry.
From
Experiment Calorimetry Calorimetry is the science of measuring heat flow. There are two types of calorimetry experiments you need to know: Calorimetry is used to measure amounts of heat transferred to or. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. 30% of the energy released during the combustion is. For example,. Experiment Calorimetry.
From
Experiment Calorimetry Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. There are two types of calorimetry experiments you need to know: Calorimetry is the science of measuring heat flow. To determine the enthalpy, stability, heat capacity One technique we can use to measure the amount of. Experiment Calorimetry.
From
Experiment Calorimetry In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a. There are two types of calorimetry experiments you need to know: One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To. Experiment Calorimetry.
From
Experiment Calorimetry To determine the enthalpy, stability, heat capacity Experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a. (1) what is the purpose of this experiment? Calorimetry. Experiment Calorimetry.
From www.youtube.com
Calorimetry Part II Lab Version) YouTube Experiment Calorimetry To determine the enthalpy, stability, heat capacity Enthalpy changes of reactions in solution. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. 30% of the energy released during the combustion is. A calorimeter is a device used to measure the amount of heat involved in. Experiment Calorimetry.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Experiment Calorimetry Enthalpy changes of reactions in solution. In a calorimetry experiment, 2.50 g of methane is burnt in excess oxygen. Calorimetry is used to measure amounts of heat transferred to or. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Experiment 6 ∙ calorimetry pre‐lab questions. Experiment Calorimetry.
From
Experiment Calorimetry To determine the enthalpy, stability, heat capacity There are two types of calorimetry experiments you need to know: 30% of the energy released during the combustion is. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a. Enthalpy changes of reactions in solution. Calorimetry. Experiment Calorimetry.
From
Experiment Calorimetry Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. There are two types of calorimetry experiments you need to know: To determine the enthalpy, stability, heat. Experiment Calorimetry.
From
Experiment Calorimetry There are two types of calorimetry experiments you need to know: (1) what is the purpose of this experiment? Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. To determine the enthalpy, stability, heat capacity Calorimetry is used to measure amounts of heat transferred to or. For example, when an. Experiment Calorimetry.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Experiment Calorimetry To determine the enthalpy, stability, heat capacity 30% of the energy released during the combustion is. Experiment 6 ∙ calorimetry pre‐lab questions answer these questions and hand them to the tf before beginning work. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. Enthalpy changes of reactions in solution. One technique we can use to. Experiment Calorimetry.
From
Experiment Calorimetry To determine the enthalpy, stability, heat capacity In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a. Calorimetry is used to measure amounts of heat transferred to or. One technique we can use to measure the amount of heat involved in a chemical or. Experiment Calorimetry.